Session Information
Date: Tuesday, November 14, 2023
Title: (1827–1839) Fibromyalgia & Other Clinical Pain Syndromes Poster
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
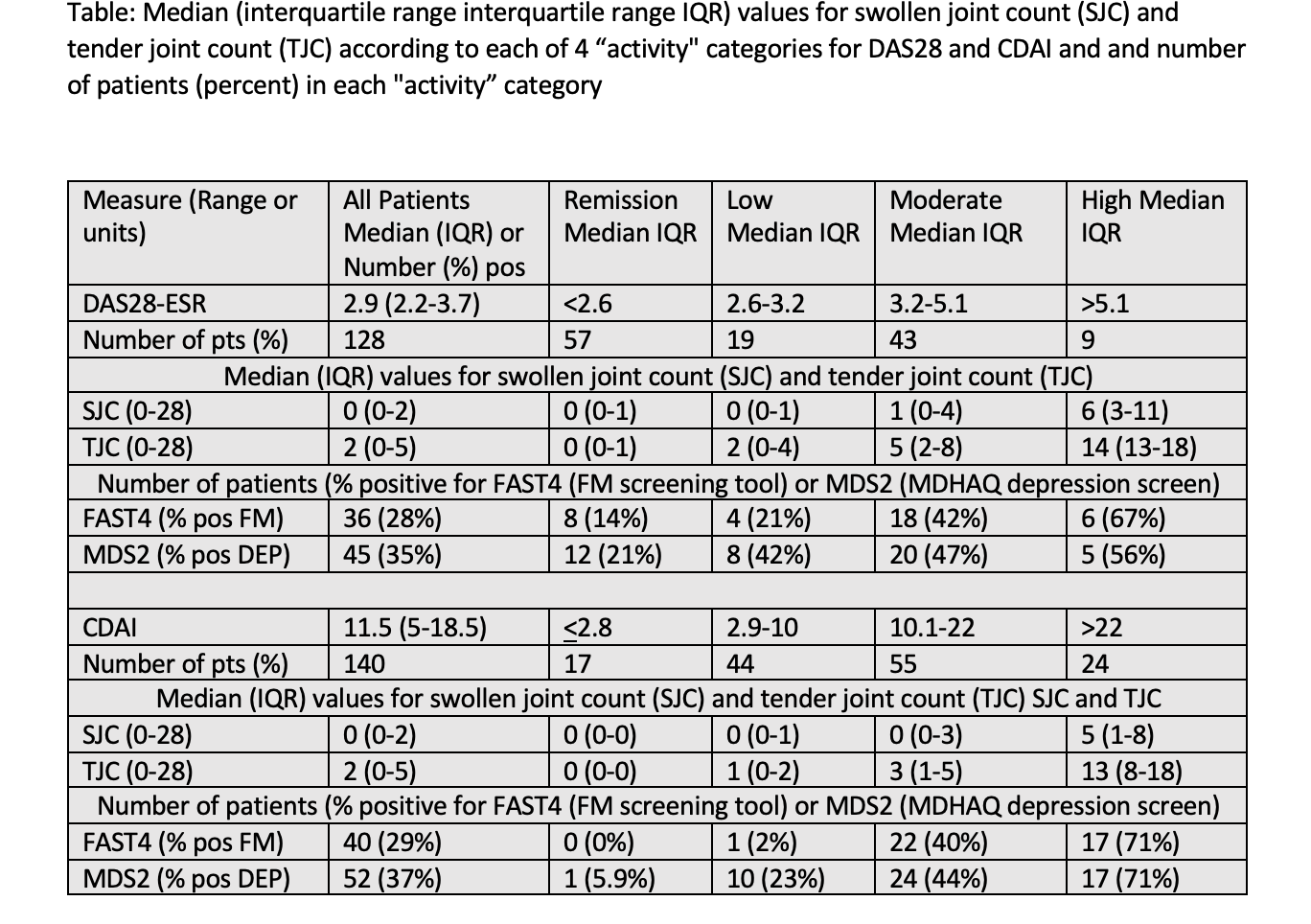
Background/Purpose: DAS28 (disease activity score 28) and CDAI (clinical disease activity index) are prominent indices to assess patients with rheumatoid arthritis (RA) quantitatively, for which disease activity is classified into 4 categories of high, moderate, low, and remission. Both DAS28 and CDAI may be elevated by non-inflammatory comorbid fibromyalgia (FM) and depression (DEP), unrelated to disease activity.Prospective systematic comparison of the number of patients in different DAS28 and CDAI activity categories who screen positive for FM and/or DEP has not been reported. Feasible screening for FM and DEP is available using MDHAQ (multidimensional health assessment questionnaire) indices for FM FAST4 (FM assessment screening tool) and DEP MDS2 (MDHAQ depression screen), which agree more than 80% with reference standards and require a single MDHAQ completed by most patients in 5-10 minutes.
Methods: Across-sectional study at a routine care visit included collection of DAS28 and CDAI.Patients completed an MDHAQ to record global assessment (PaGA), pain, and fatigue on 0-10 visual numeric scales (VNS), a 0-3 DEP query, 0-54 self-report RADAI painful joint count, 60-symptom checklist including DEP, and medical history queries. A FAST4 FM screen is positive if 3/4 are met: pain VNS≥6/10, fatigue VNS≥6/10, RADAI ≥16/54, symptom checklist≥16/60. An MDS2 DEP screen is positive if 0-3.3 DEP response is ≥2.2 OR positive DEP on the symptom checklist. Patients were classified into 4 DAS28-ESR and CDAI activity categories: high ( >5.1/28 & >22/76), moderate (3.2-5.1 & 10.1-22), low (2.6-3.2 & 2.9-10), and remission (≤2.6 & 2.8), respectively. The median swollen and tender joint counts (SJC and TJC) and proportions of FAST4 and MDS2 patients in each category were compared using chi-square analyses.
Results: Among 128 patients for DAS28 and 140 for CDAI (12 were missing ESR), categories of remission, low, moderate, and high activity, respectively, included 44%, 15% 34%, and 7% by DAS28-ESR, and 13%, 31%, 39% and 16% by CDAI. Median SJC was 0, 0, 2 and 4 for patients in DAS28-ESR and 0, 0, 0 and 4 for patients in CDAI categories. Median TJC was 0, 2, 5 and 5 in 4 DAS28-ESR categories, and 0, 1, 3 and 14 in 4 CDAI categories, respectively (Table). Positive FAST4 FM screen was seen in 28-29% and MDS2 screen in 35-37% of all patients.Positive FAST4 screen was seen in 13%, 22%, 44% and 67% in 4 DAS28-ESR categories of remission, low, moderate, and high activity, respectively, and 0%, 2.6%, 38%, and 80% in the 4 CDAI categories, respectively. Positive MDS2 DEP was seen in 20%, 44%, 46% and 56% in 4 DAS28-ESR categories, and 6%, 24%, 40% and 70% in 4 CDAI categories, respectively.
Conclusion: Among RA patients classified as in high DAS28-ESR or CDAI activity, more than 50% screened positive for FM or DEP, compared to lower proportions for more favorable categories, including fewer than 21% for those in DAS28-ESR or CDAI remission.These phenomena may affect treat-to-target and other aspects of RA management. Feasible screening for FM and DEP is available according to valid MDHAQ FAST4 and MDS2 indices, as well as RAPID3 (routine assessment of patient index data), requiring only a single questionnaire which is completed by most patients in 5-10 minutes.
To cite this abstract in AMA style:
Pincus T, Hunter R, Rodwell N. Positive Screening for Fibromyalgia or Depression on Validated MDHAQ Indices Is Seen in 56-71% of Rheumatoid Arthritis Patients with High DAS28 or CDAI, 40-47% with Moderate, 2-42% with Low, and 0-21% with DAS28 or CDAI Remission [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/positive-screening-for-fibromyalgia-or-depression-on-validated-mdhaq-indices-is-seen-in-56-71-of-rheumatoid-arthritis-patients-with-high-das28-or-cdai-40-47-with-moderate-2-42-with-low-and-0-21-wit/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/positive-screening-for-fibromyalgia-or-depression-on-validated-mdhaq-indices-is-seen-in-56-71-of-rheumatoid-arthritis-patients-with-high-das28-or-cdai-40-47-with-moderate-2-42-with-low-and-0-21-wit/