Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Atacicept targets the B-cell stimulating factors BLyS and APRIL and has been shown to reduce SLE disease activity. The aim of the analysis was to describe the pharmacokinetic (PK) profile of total (i.e. bound and unbound) atacicept after subcutaneous (sc) administration in healthy volunteers (HV) and SLE patients using a semi-mechanistic population PK model and to identify covariates explaining PK variability.
Methods: A total of 540 subjects, 37 from a Phase I study in Caucasian and Japanese HV where single 25, 75 or 150 mg sc doses of atacicept were administered, 298 from a Phase II study in SLE (NCT00624338) where 75 or 150 mg sc doses were administered weekly (QW) for 52 weeks (bi-weekly for the first 4 weeks of treatment), and 205 from a second Phase II study in SLE (NCT01972568) with QW sc dosing of 75 or 150 mg for 24 weeks, contributed 3640 measurements of total atacicept in serum. Modeling was performed with the NONMEM software. A Quasi-Steady-State (QSS) approximation [1] of the target-mediated drug disposition (TMDD) [2] model was used to describe drug concentrations. Covariates tested in the model were weight, age, creatinine clearance, serum BLyS and APRIL (all at baseline), gender, race, dose and SLE vs HV population. Model-based exposure metrics (e.g. area under the concentration curve, AUC) were derived. Covariate effects were evaluated via simulations.
Results: A two-compartment QSS TMDD binding model with first-order absorption described total atacicept concentrations of the three trials, adequately capturing the central tendency and variability in the data. The model provided precise (relative standard errors < 20%) estimates of all parameters, including binding (Kss=19.9 ng/mL), target turnover (Rmax=715 ng/mL; Kdeg=0.00362 h-1), and drug-target complex elimination (Kint=0.000618 h-1) parameters. The typical estimates of apparent linear clearances and volumes of distribution of the drug were: CL/F=0.324 L/h, Vc/F=36.3 L, Q/F=0.149 L/h and Vp/F=38.5 L. Residual variability was moderate, slightly higher in SLE patients (CV=25%) than in HV (CV=19%).
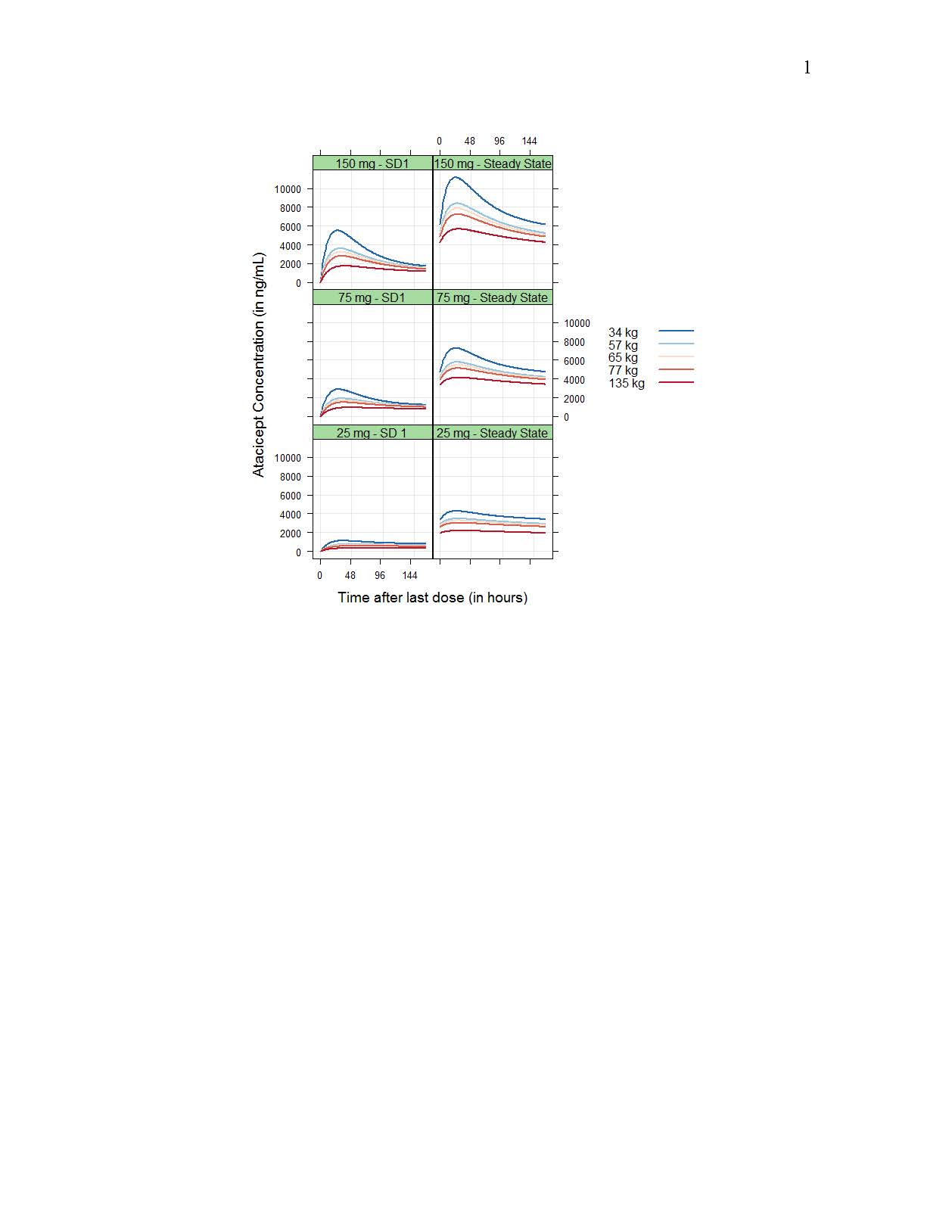
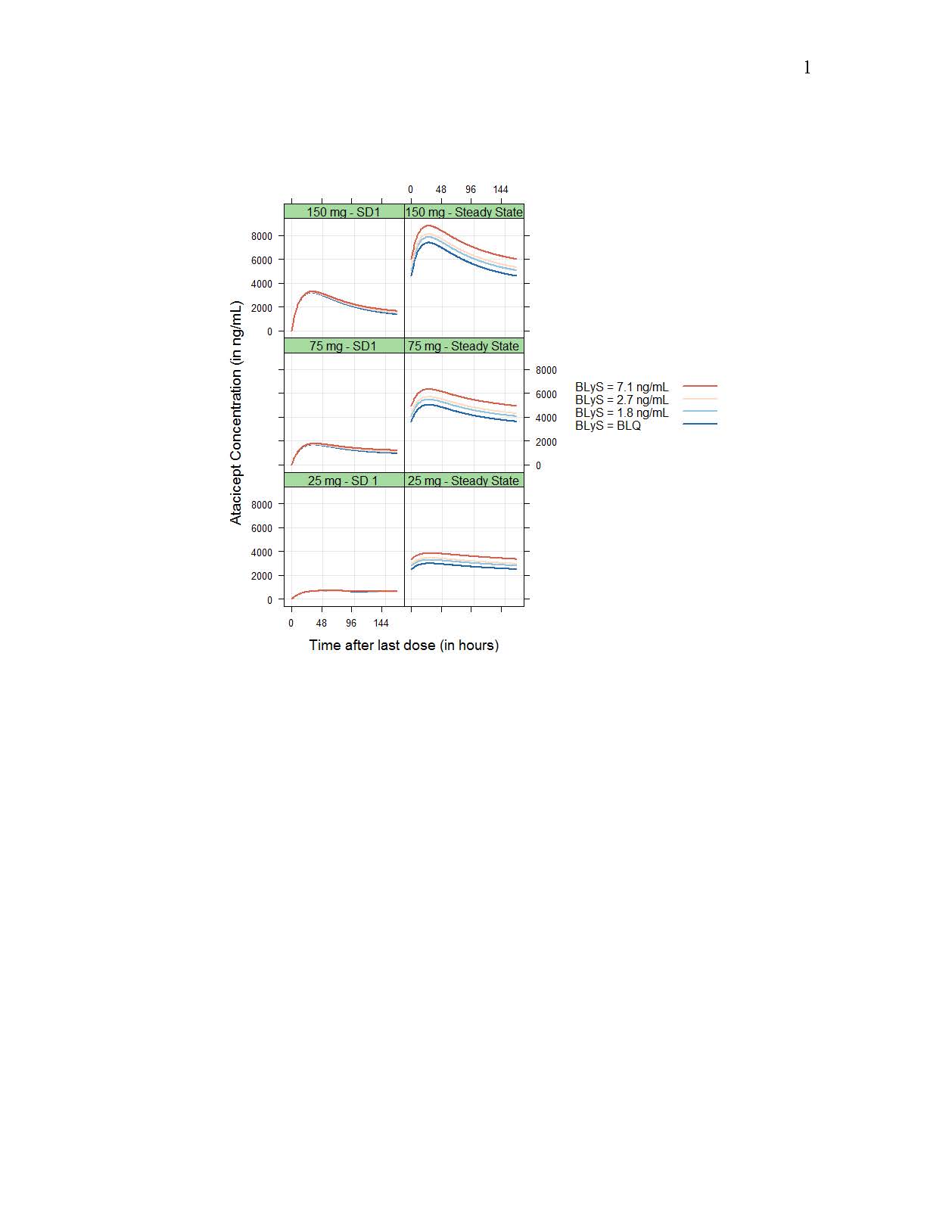
Drug CL/F and central volume Vc/F increased with body weight following allometric relationships (exponents of 0.75 and 1.00, respectively), while baseline target concentration (Rmax) increased with baseline BLyS concentration (as Rmax ~(BLyS/2.56)0.176); however, resulting differences in exposure were small (Figure 1 and 2). No significant differences in PK between HV and SLE patients or racial groups were detected.
Conclusion: The developed population PK model allowed the description of the complete atacicept concentration time profile in SLE patients, a first step in the identification of exposure-response relationships for pharmacodynamic/clinical/safety endpoints that informed on Phase III study design.
References:
[1] Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn, 2008; 35:573-591.
[2] Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn, 2001; 28:507-532.
To cite this abstract in AMA style:
Pitsui M, Papasouliotis O, Farrell C, Girard P, Yalkinoglu O, Vazquez-Mateo C. Population Pharmacokinetics of Atacicept in Systemic Lupus Erythematosus (SLE) – an Analysis of Three Clinical Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/population-pharmacokinetics-of-atacicept-in-systemic-lupus-erythematosus-sle-an-analysis-of-three-clinical-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/population-pharmacokinetics-of-atacicept-in-systemic-lupus-erythematosus-sle-an-analysis-of-three-clinical-trials/