Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background/Purpose: It has been well investigated that patients with lupus nephritis (LN) have worse prognosis than those without. Recently reported, about 20% of SLE patients without abnormal urinalysis have histopathologically proven lupus nephritis, so-called silent LN. Here, we investigated long-term renal outcome in SLE patients without abnormal urinalysis and additionally determined whether their renal outcomes correlates with abnormal B lymphocyte activity in circulation.
Methods: Methods: We retrospectively evaluated newly diagnosed SLE patients from 2000 to 2018 in our hospital. All the patients were divided them into 2 groups according to the presence of abnormal urinalysis during the observation. Abnormal urinalysis was defined as a persistent proteinuria (more than 50 mg/dL or 0.5 g/gCr for more than 3 months). Deterioration of renal function (more than 40% eGFR decline from baseline) was compared between them. For the patients without abnormal urinalysis, the distribution of B cell subsets was compared by fluorescence-activated cell sorting analysis using anti-CD19, 20, 27, 38, 138, and IgD antibodies depending on the deterioration of renal function.
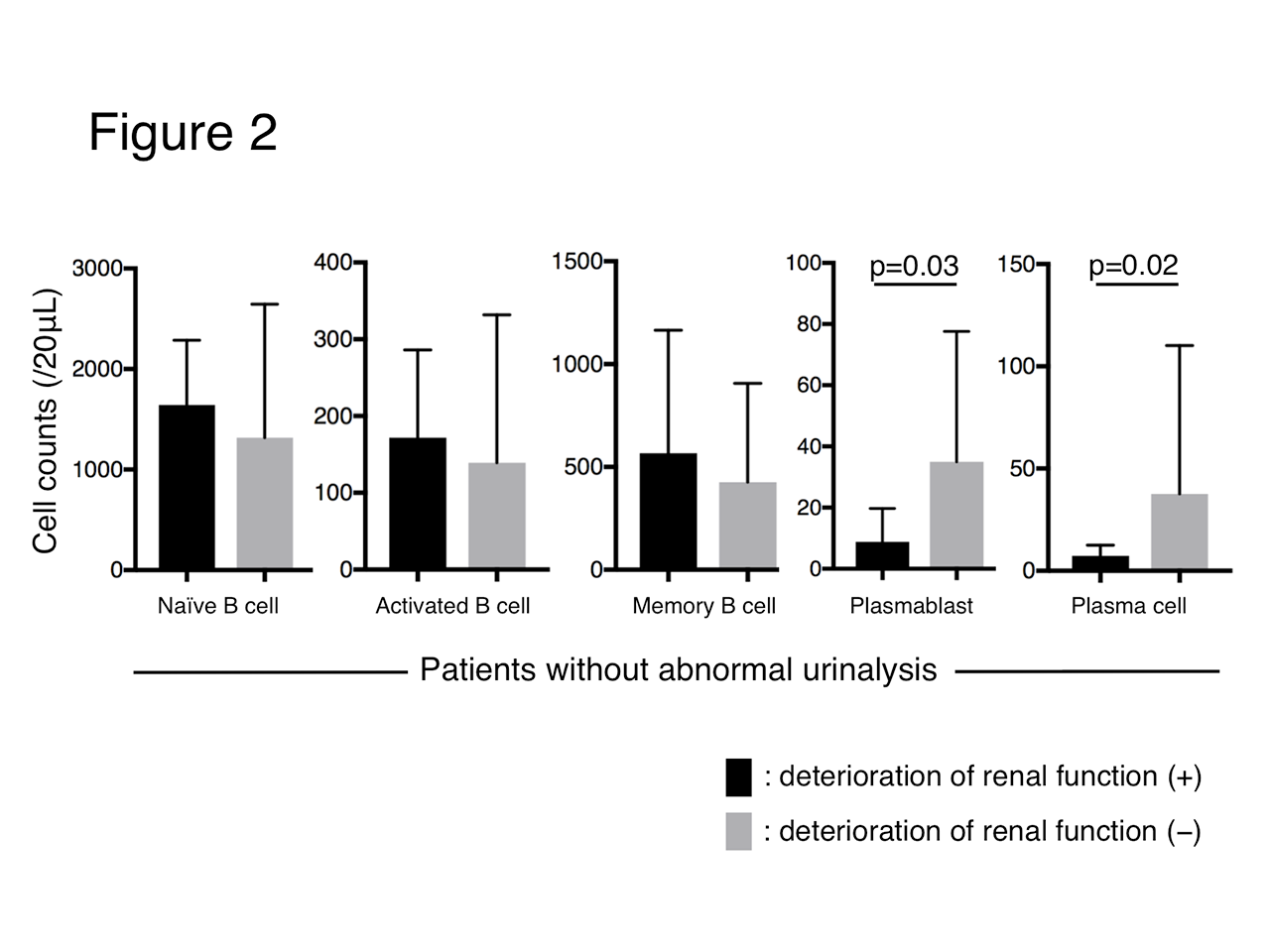
Results: Results: Seventy-three patients with abnormal urinalysis and 62 without were evaluated. Among patients with abnormal urinalysis, 62 (84.9%) had biopsy-proven LN. Patients with abnormal urinalysis had a higher titer of anti-dsDNA antibodies (p< 0.01), SLEDAI score (p< 0.01), maximum dose of prednisolone (p=0.04), percentage of IVCY use (p< 0.01) and MMF use (p< 0.01) than those without. There was no significant difference in observational periods (10.1 vs 11.6 years, p=0.43) and cumulative deterioration rate of renal function (19.7 vs 16.1%, p=0.31) between them (Figure 1). For patients without abnormal urinalysis, a higher positivity of anti-Sm antibody (70.0% vs 5.0%, p< 0.01) and a lower count of plasmablast (CD19+/27+/IgD+/38+/138−) (p=0.03) and plasma cell (CD138+) (p=0.02) were observed in patients with deteriorated renal function than those without (Figure 2).
Conclusion: Conclusion: Long-term renal outcome was not significantly different between patients with and without abnormal urinalysis for about 10-years observation. Positivity of anti-Sm antibody and decreased number of peripheral plasmablast and plasma cell might be surrogate markers for deterioration of renal function in patients without abnormal urinalysis. Silent LN might contribute to our results and further analysis performing renal biopsy is needed.
in patients without abnormal urinalysis for deterioration of renal function.
To cite this abstract in AMA style:
Hanaoka H, Kikuchi J, Saito S, Takei H, Hiramoto K, Oshige T, Seki N, Tsujimoto H, Kaneko Y, Takeuchi T. Poor Long-term Renal Outcome in Systemic Lupus Erythematosus Without Abnormal Urinalysis: A Possible Link with Silent Lupus Nephritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/poor-long-term-renal-outcome-in-systemic-lupus-erythematosus-without-abnormal-urinalysis-a-possible-link-with-silent-lupus-nephritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/poor-long-term-renal-outcome-in-systemic-lupus-erythematosus-without-abnormal-urinalysis-a-possible-link-with-silent-lupus-nephritis/