Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
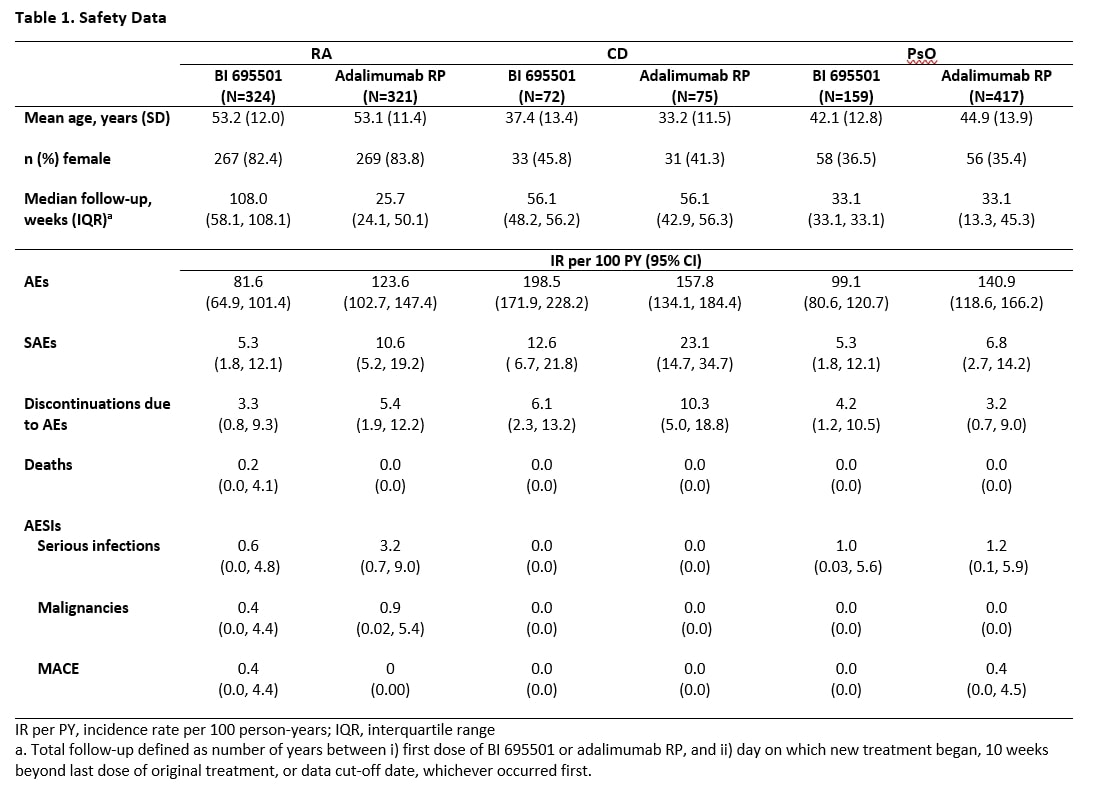
Background/Purpose: The VOLTAIRE trials program compared the safety, efficacy, and immunogenicity of biosimilar BI 695501 with adalimumab reference product (RP) for indications including moderate-severe rheumatoid arthritis (RA), Crohn’s disease (CD), and chronic plaque psoriasis (PsO),1-4 and included a switching study to investigate interchangeability.5 This analysis estimates the incidence of safety endpoints across 5 phase 3 randomized controlled clinical trials in patients with RA (VOLTAIRE-RA and VOLTAIRE-RAext), CD (VOLTAIRE-CD), and PsO (VOLTAIRE-PsO and VOLTAIRE-X) who received ≥1 dose of BI 695501 or adalimumab RP.
Methods: Safety endpoints included in this analysis were adverse events (AEs), serious adverse events (SAEs), discontinuations due to AEs, deaths, and adverse events of special interest (AESIs; specifically, serious infections, malignancies, and major adverse cardiovascular events [MACE; defined as non-fatal stroke, myocardial infarction, or fatal cardiovascular death]). Exposure-adjusted incidence rates (EAIR) were calculated per 100 patient-years. Incidence rates are reported by disease indication and treatment arm. For the at‐risk person‐years, the starting point was the first dose of BI 695501 or adalimumab RP, and the end point was 10 weeks after the last dose, the data cut‐off date or the first safety event (whichever occurred first). Subgroup analyses by patient age and sex were also conducted.
Results: The mean age of patients with RA was higher than that of patients with CD or PsO, and a greater proportion of patients with RA were female. The mean length of follow up in this analysis was 62 weeks in patients with RA, 48 weeks in patients with CD, and 32 weeks in patients with PsO. Safety data are summarized in Table 1. Rates of SAEs and discontinuations due to AEs were similar among patients with RA and PsO, but slightly higher among those with CD. Overall incidence rates of AEs, SAEs, discontinuations due to AEs, deaths, and AESIs were consistent between the BI 695501 and adalimumab RP treatment arms within each indication. There were no cases of malignancies or MACE reported in patients with PsO or CD, while both were observed in patients with RA. Subgroup analyses of patients with RA by age and sex showed no between-group differences, and a similar trend was observed for patients with CD and PsO.
Conclusion: In patients with RA, CD and PsO, there were no differences between biosimilar BI 695501 or the adalimumab RP regarding the rate of AEs, SAEs, discontinuations due to AEs, deaths, or the AESIs of serious infections, cancer, or MACE. Trial Registrations: VOLTAIRE-RA, NCT02137226; VOLTAIRE-RAext, NCT02640612; VOLTAIRE-CD, NCT02871635; VOLTAIRE-PsO, NCT02850965
To cite this abstract in AMA style:
Cohen S, Bender S, Shaberman A, Vinisko R, McCabe D. Pooled Safety Analysis from the VOLTAIRE Trials in Patients with Rheumatoid Arthritis, Crohn’s Disease, and Chronic Plaque Psoriasis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/pooled-safety-analysis-from-the-voltaire-trials-in-patients-with-rheumatoid-arthritis-crohns-disease-and-chronic-plaque-psoriasis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pooled-safety-analysis-from-the-voltaire-trials-in-patients-with-rheumatoid-arthritis-crohns-disease-and-chronic-plaque-psoriasis/