Session Information
Date: Wednesday, November 11, 2015
Title: Pain: Clinical Aspects
Session Type: ACR Concurrent Abstract Session
Session Time: 11:00AM-12:30PM
reduces pain and improves physical function and Patient’s Global Assessment
(PGA) in patients with chronic pain. From June 2010 to August 2012 the US FDA imposed
a partial clinical hold on noncancer pain-related studies due to unexpected
adverse events initially reported as osteonecrosis that required total joint
replacement.
clinical trials of TNZ in patients with moderate-to-severe OA of the knee or
hip completed before the clinical hold were pooled to evaluate efficacy and 9 phase
3 controlled OA studies were pooled to evaluate safety. Patients received 1 to 3
injections of intravenous TNZ 2.5, 5, or 10 mg every 8 weeks, naproxen 500 mg twice
daily (BID), celecoxib 100 mg BID, oxycodone controlled release 10-40 mg BID, or
PBO. Efficacy was assessed using WOMAC pain and WOMAC physical function
subscales and PGA of OA as co-primary endpoints; ≥30%, ≥50%,
≥70%, and ≥90% improvement on WOMAC Pain subscale were secondary
endpoints. Safety assessments included adverse event documentation and physical
and neurologic examinations. Patients who reported abnormal peripheral
sensation and/or had clinically significant neurologic exam findings underwent
neurologic consultation. Subgroup analyses were also conducted on the pooled
populations.
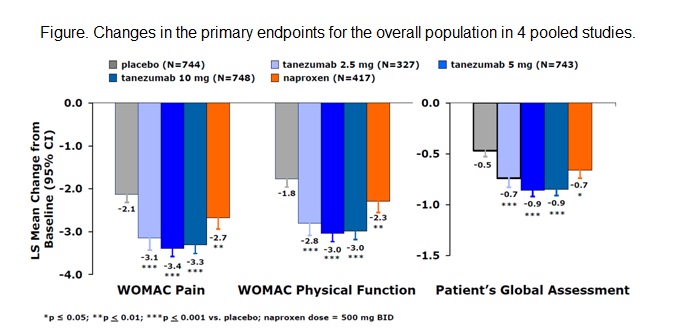
mg provided significant improvement in all efficacy endpoints (p≤0.05;
Figure). More patients treated with TNZ 5 or 10 mg reported pain improvement ≥30%,
≥50%, ≥70%, and ≥90% (p≤0.05 for all). Substantial
increases in efficacy were not noted for TNZ 10 mg over TNZ 5 mg. Incidence of
adverse events in TNZ-treated patients was similar to patients receiving active
comparator and increased over PBO-treated patients; rates with TNZ 5 and 10 mg were
similar and elevated vs TNZ 2.5 mg. Adverse events of abnormal peripheral
sensation were reported more frequently by patients receiving TNZ vs PBO or
active comparator. Most TNZ-treated patients whose final neurologic
consultations were categorized as having a new or worsening peripheral
neuropathy based on clinically significant signs or diagnostic tests were
diagnosed with some form of mononeuropathy, predominantly carpal tunnel
syndrome or radiculopathy; few patients were diagnosed with a polyneuropathy. TNZ
10 mg but not 2.5 or 5 mg was associated with a higher rate of rapidly
progressive OA than active comparator.
of pain, physical function, and PGA of OA. Non-joint-related safety was similar
in patients treated with TNZ 2.5-10 mg versus active comparator but increased versus
PBO-treated patients.
Disclosure: L. Tive, Pfizer Inc, 3,Pfizer Inc, 1; D. Radin, None; A. Bello, None; H. Nguyen, Pfizer Inc, 1,Pfizer Inc, 3; M. T. Brown, Pfizer Inc, 1,Pfizer Inc, 3; C. R. West, Pfizer Inc, 1,Pfizer Inc, 3; K. M. Verburg, Pfizer Inc, 1,Pfizer Inc, 3.
To cite this abstract in AMA style:
Tive L, Radin D, Bello A, Nguyen H, Brown MT, West CR, Verburg KM. Pooled Efficacy and Safety from Phase 3 Controlled Studies of Tanezumab in Patients with Osteoarthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/pooled-efficacy-and-safety-from-phase-3-controlled-studies-of-tanezumab-in-patients-with-osteoarthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pooled-efficacy-and-safety-from-phase-3-controlled-studies-of-tanezumab-in-patients-with-osteoarthritis/
