Session Information
Date: Sunday, November 12, 2023
Title: (0283–0307) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Pneumocystis Jirovecii Pneumonia (PJP) is an opportunistic fungal infection with high morbidity and mortality rates. Few studies to date have assessed the incidence of PJP among patients initiating disease-modifying antirheumatic drugs (DMARDs) for idiopathic inflammatory myopathies. The objective of this study was to determine the incidence of PJP among patients receiving treatment for idiopathic inflammatory myopathies.
Methods: Data were derived from the US-based electronic health records database, TrinetX. Patients selected for study inclusion if they had greater than or equal to 2 ICD9-CM or ICD10-CM diagnosis codes for myositis and received at least one prescription for a DMARD . Due to the retrospective and electronic medical records based nature of this study, items from the ACR Classification Criteria were not available. PJP diagnosis was defined as hospitalization with diagnosis of PJP, any PJP code followed by any PJP treatment, or any diagnostic test positive for PJP. The primary outcome was the incidence of PJP during the first 6 months of initiating DMARD therapy. The secondary outcome was the incidence of PJP during the first 12 months of initiating DMARD therapy. Unadjusted incidence rate ratio and 95% confidence intervals were calculated.
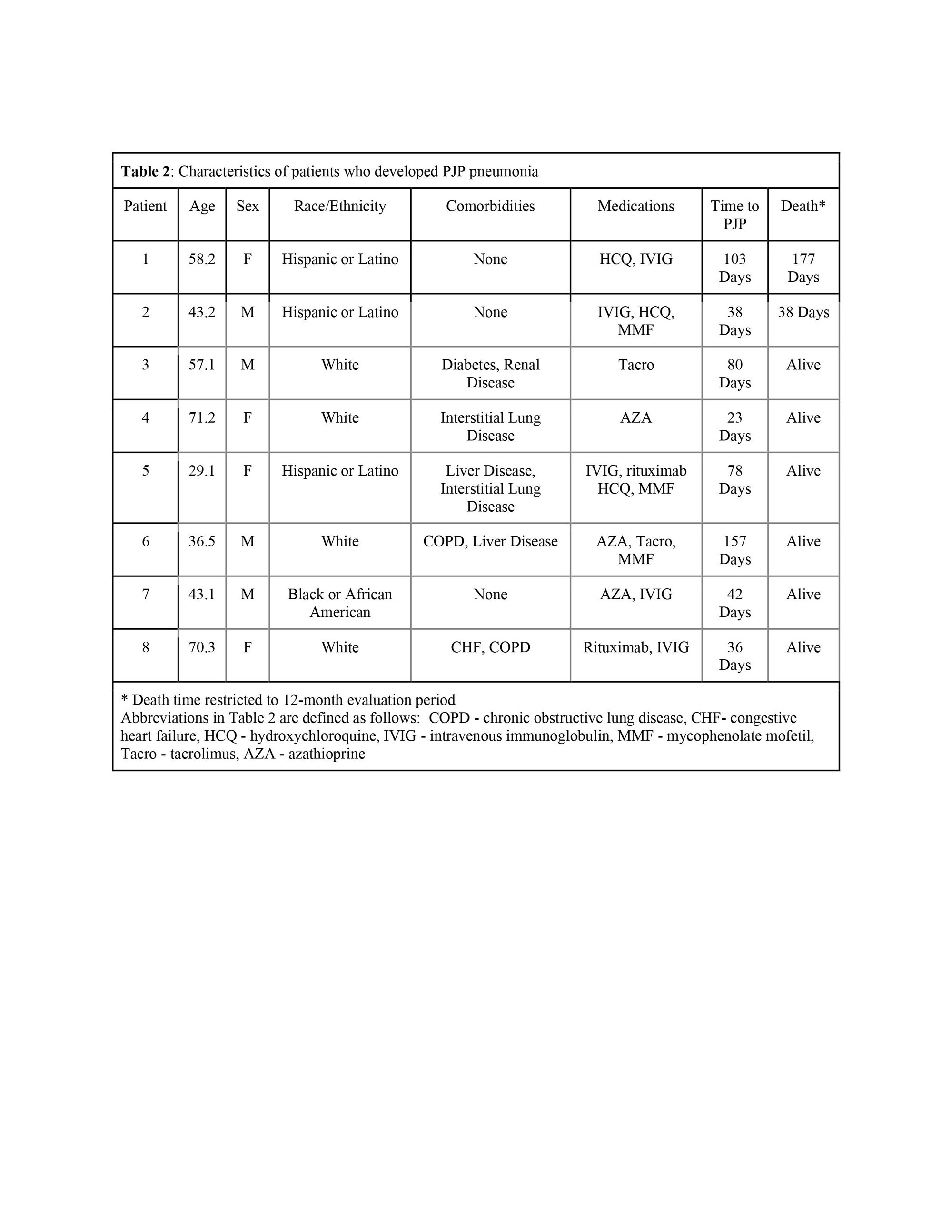
Results: This study identified 6,030 patients with myositis who initiated therapy with a DMARD, the most common being methotrexate (2,826, 46.9%), followed by azathioprine (1,531, 25.4%) and mycophenolate mofetil (1,482 ,24.6%). The majority of patients were female (4,532, 75.2%) and white (3,997, 66.3% ). The average age at diagnosis of myositis was 55.0 years. A minority (982, 16%) of patients received PJP prophylaxis, the most common of which was trimethoprim/sulfamethoxazole (852, 14.1%). During the first six months of therapy after DMARD initiation, a total of 8 cases of PJP were identified for an incidence rate of 2.9 cases per thousand patient-years. Among the eight patients who developed PJP, four were female, the average age at diagnosis was 51.1 (SD 15.5 years), and 3 had received prophylaxis against PJP. The average time to PJP diagnosis from the index date was 69.6 days (SD 44.7 days). The incidence of PJP among patients with structural lung disease at baseline (defined by chronic obstructive pulmonary disease or interstitial lung disease) was numerically higher as compared to those without lung disease (incidence 5.3 cases vs. 2.0 cases per 1,000 person) with an incident rate ratio of 2.7, 95% (confidence intervals 0.67-10.67). After extending the evaluation period to 1 year after DMARD initiation, no additional cases of PJP were identified, so the 12-month incidence of PJP was 1.45 cases per thousand patient-years.
Conclusion: The incidence of PJP in patients receiving DMARDs for idiopathic inflammatory myopathy was lower than identified in previous literature. It is important to weigh this low incidence rate against potential harms of antimicrobial therapy when deciding to initiate PJP prophylaxis. The risk of PJP in this population may be elevated among patients with structural lung disease.
To cite this abstract in AMA style:
Bruggemeyer C, Shah S, Saygin D, Putman M. Pneumocystis Jirovecii Pneumonia in Patients with Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/pneumocystis-jirovecii-pneumonia-in-patients-with-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pneumocystis-jirovecii-pneumonia-in-patients-with-inflammatory-myopathies/