Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sjogren’s Syndrome (SS) is a chronic autoimmune disease characterized by keratoconjunctivitis sicca and xerostomia. Currently there is no approved systemic treatment for primary SS. The purpose of this study was to determine the safety and effect of the drug ustekinumab on patients with pSS. Ustekinumab, a humanized monoclonal antibody, works by inhibiting the p40 subunit of the proteins IL-12 and IL-23. Ustekinumab has been FDA approved for patients with psoriasis, psoriatic arthritis, and crohn’s disease and has been shown to be a safe and therapeutic treatment for these conditions.
The primary clinical objective was to examine the safety of ustekinumab in patients with pSS.
The primary mechanistic objective was to determine whether the standard dosing schedule of ustekinumab lowers serum biomarkers of inflammation in patients with pSS.
Secondary objectives were to assess the effect of ustekinumab on the subjective and objective clinical manifestations of pSS.
Methods: We performed a prospective, single-center, open-label pilot trial.
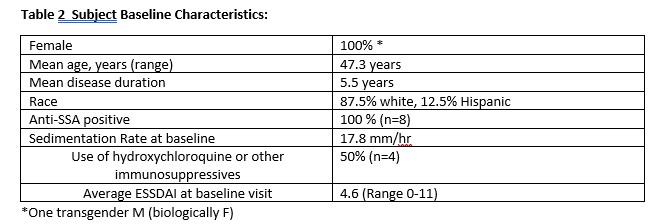
All patients gave written informed consent to participate. The study protocol was approved by University of Rochester IRB and Jannsen Scientific Affairs. A total of 12 patients were recruited. 4 screen failed. 8 patients completed the study. Baseline characteristics of the patients are shown in Table 2.
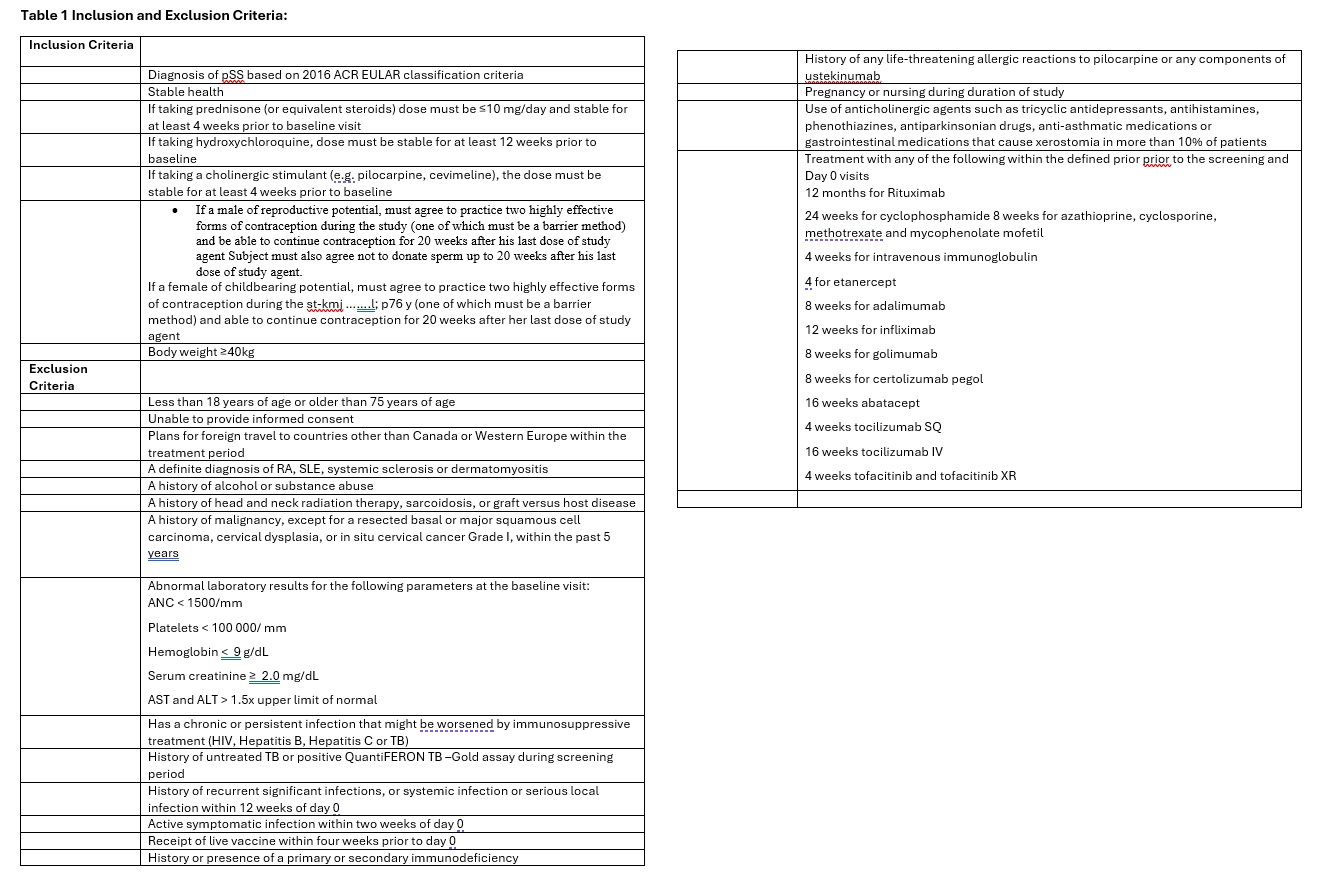
Inclusion and Exclusion criteria are in Table 1. Patients had to be between ages of 18-75 years of age and had to fulfill 2016 ACR/EULAR Sjogren’s Classification Criteria.
Results: Ustekinumab was well tolerated by all study participants. There were no interruptions in therapy. Mild adverse events included nausea, increased reflux, mild rashes thought to be unrelated to study drug. There were no serious infections. No serious adverse events resulting in hospitalizations.
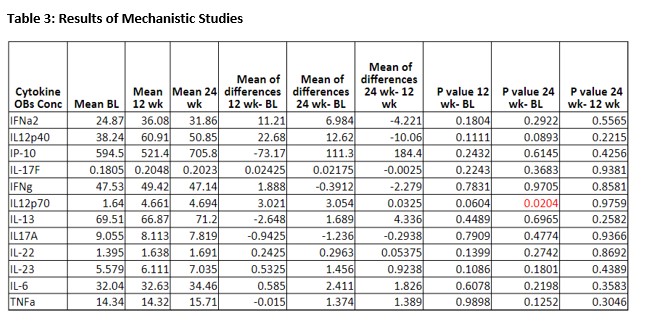
Results of cytokine concentration through baseline to week 24 are shown in Table 3. There was no statistically significant change in cytokine signatures between weeks 0 and 24 except for IL12p70 which is likely secondary to mechanism of ustekinumab. There was no statistically significant change in level of IFN, IL-17, IL-6 or TNF-alpha.
Conclusion: No significant adverse events were identified in this study.
We did not recruit goal of 15 patients. Given the limited sample size it was difficult to assess effect of ustekinumab on clinical and mechanistic measures. Of the mechanistic studies there was a significant change in IL12p70 level. This is likely secondary to mechanism of ustekinumab but unclear if this has any clinically meaningful effect in this population. No significant change was seen in laboratory measures of serum disease activity.
The results of this study demonstrate relative safety of treatment with ustekinumab for Sjogren’s Syndrome. There was not a significant clinical change in sicca complex, but this may be further investigated in larger studies.
To cite this abstract in AMA style:
Shah U, Coca A. Pilot Trial of Ustekinumab for Primary Sjogren’s Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pilot-trial-of-ustekinumab-for-primary-sjogrens-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pilot-trial-of-ustekinumab-for-primary-sjogrens-syndrome/