Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The Type 1 & 2 SLE Model was developed to better explain the signs, symptoms, and management goals of SLE to patients. We assembled tools to discuss the Type 1 & 2 SLE Model, including patient-reported outcome measures, physician global assessment of disease activity (PGA) scores for Type 1 and Type 2 SLE activity, EMR documentation shortcuts, and a patient handout. In this pilot study, we aimed to guide the implementation of these tools in rheumatology care outside of our lupus clinic with the goal of increasing the frequency of discussion of the Type 1 & 2 SLE Model.
Methods: We conducted a 4-week study at an academic rheumatology clinic. Participating providers received training on the Type 1 & 2 SLE tools during an interactive presentation that reviewed each of the tools, summarized the approach to treating Type 2 SLE symptoms, and case examples of scoring PGAs. During the intervention period, all SLE patients of participating providers received a questionnaire at check-in that included the Systemic Lupus Activity Questionnaire (SLAQ) and the ACR Fibromyalgia Severity Score. At the end of each visit, patients completed a brief, anonymous satisfaction survey. Providers completed baseline and follow-up surveys that asked about satisfaction with care and the acceptability, appropriateness, and feasibility of the intervention. Clinic notes of patients with SLE seen during the intervention period and 4-weeks prior to the intervention period were reviewed for discussions of the Type 1 & 2 SLE Model.
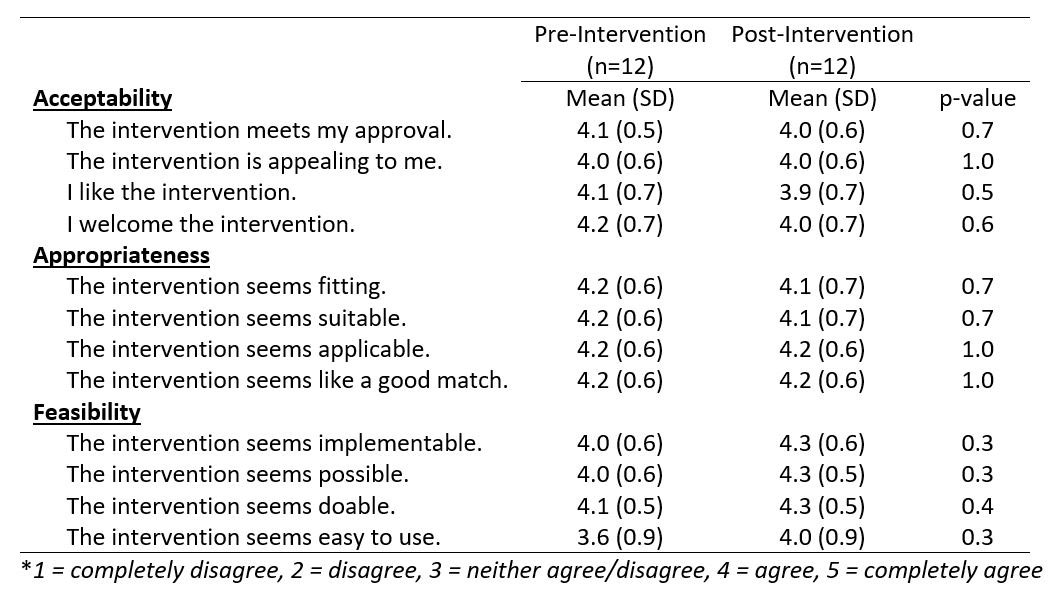
Results: Sixteen of 25 eligible providers participated (3 APPs, 8 faculty, 5 fellows); 36 patients with SLE were seen in the pre-intervention period (mean age 50, 83% female, 60% Black) and 31 patients were seen during the intervention period (mean age 49 years, 87% female, 55% Black). Provider surveys were completed by 12 participants at baseline and 12 participants at follow-up. At follow-up, provider surveys showed high scores for acceptability (4.0/5), appropriateness (4.15/5), and feasibility (4.2/5) of the intervention (Table 1). All providers agreed or completely agreed the intervention seemed possible, and there was an increase in the proportion who felt the intervention was easy to use (50% to 83%).
The Type 1 & 2 PGAs were documented in 87% of notes. The discussion of Type 2 SLE symptoms increased from 44% to 74% of patients (p=0.02). Discussing the Type 1 & 2 SLE Model did not increase the duration of clinic visits during the intervention period (Table 2).
Among 49 patients who completed surveys (19 pre-intervention and 30 intervention), satisfaction with care remained high (Table 3). Almost all (80-95%) patients strongly agreed they felt good about their visit, they and their rheumatologist agreed on their SLE activity, their rheumatologist gave their full attention, they were able to say everything they wanted and they understood their care recommendations.
Conclusion: By implementing the Type 1 & 2 SLE Model, our general rheumatologists increased their discussion of Type 2 SLE symptoms without significantly increasing the duration of clinic visits. All patients remained highly satisfied with their care. Future work will take this intervention to other rheumatology clinics to determine its impact on patient outcomes.
To cite this abstract in AMA style:
Eudy A, Rogers J, Sun K, Sadun R, Pisetsky D, Maheswaranathan M, Doss J, Criscione-Schreiber L, Clowse M. Pilot Study to Implement the Type 1 & 2 SLE Model in an Academic Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/pilot-study-to-implement-the-type-1-2-sle-model-in-an-academic-rheumatology-clinic/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pilot-study-to-implement-the-type-1-2-sle-model-in-an-academic-rheumatology-clinic/