Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Endothelial dysfunction is present in arthritis animal models and rheumatoid arthritis (RA), a population known to develop premature cardiovascular disease. Previous studies indicate inflammation modulates phosphorylation of endothelial nitric oxide synthase (eNOS) at the S1177 residue leading to endothelial impairment. The expression of eNOS in the RA vasculature has not been assessed. We investigated the hypothesis that subjects with high disease activity RA, defined by an abnormal C-reactive protein and disease activity score (DAS) >3.2, will have reduced levels of eNOS expression as compared to low disease activity RA patients and healthy controls.
Methods: We assessed endothelial function in 10 high disease activity RA, 14 low disease activity RA, and 14 healthy control (HC) subjects by brachial artery flow mediated dilation (FMD) and extracted venous endothelial cells by soft tipped J-wires from forearm catheters. RA subjects met the 1987 ACR diagnostic criteria and were either rheumatoid factor or anti-citrullinated peptide antibody positive. Expression of eNOS and phosphorylated eNOS (p-eNOS) was captured by digital immunofluorescence microscopy using human umbilical vein endothelial cell (HUVEC) controls.
Results: High disease activity RA subjects (mean age 44 years, 86% female) were significantly different from low disease activity (mean age 48 years, 91% female) for DAS (5.55±0.32 vs 2.36±0.16 au p=0.0001) and health assessment questionnaire (0.94±0.14 vs 0.34±0.10 au p=0.002). HC subjects (mean age of 43 years, 79% female) were found to have lower systolic blood pressure compared to high and low disease activity RA subjects (107±1.8 vs 119±3.6 vs 121±2.7 mmHg p=0.004). Body mass index was greater in high disease activity RA compared to low disease activity and HC (32±1.8 vs 26±1.5 vs 25±1.2 au respectively, p=0.008). Methotrexate and biologic medications were used with similar frequencies between RA subject groups (p=0.8 and p=0.7 respectively).
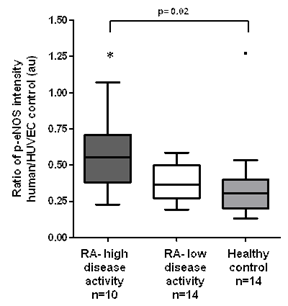
Despite higher levels of C-reactive protein in high disease activity RA (1.9±0.5 mg/dL vs 0.27±0.05, p=0.0001), we found the expression of p-eNOS to be significantly greater as compared to low disease activity RA and HC (0.58±0.08 vs 0.38±0.03 vs 0.36±0.08 au, respectively, p=0.02, see figure). eNOS expression was similar between high and low disease activity RA and HC (0.47±0.05 vs 0.54±0.06 vs 0.35±0.05 au, respectively, p=0.24). FMD percent change in dilation over baseline was similar between high and low disease activity RA and HC (8.5±1.12 vs 9.2±1.65 vs 9.3±1.10 au, respectively, p=0.99).
Conclusion: In conclusion, we show upregulation of p-eNOS expression in high disease activity RA subjects with elevated C-reactive protein. Further studies are required to better understand the molecular mechanisms of endothelial dysfunction in RA that lead to premature cardiovascular disease.
Disclosure:
E. B. Solow,
None;
W. Vongpatanasin,
None;
D. R. Karp,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phosphorylated-venous-endothelial-nitric-oxide-synthase-is-elevated-in-rheumatoid-arthritis-subjects-with-high-disease-activity/