Session Information
Date: Sunday, November 5, 2017
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment I: Novel and Current Therapies
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The Phase 3 CHABLIS-SC1 trial (NCT01395745) evaluated blisibimod, an inhibitor of B-cell activating factor (BAFF), in SLE. Prior SLE trials suggested that treatments are better distinguished from placebo in patients with higher disease activity, greater corticosteroid use, anti-double-stranded DNA (dsDNA), low complement1. The population in this study was enriched for these factors.
Methods: 442 SLE patients on corticosteroids (≤ 0.5 mg/kg or 40 mg) with anti-nuclear antibodies and/or anti-dsDNA and SELENA-SLEDAI score ≥10 on standard of care medications were randomized to weekly subcutaneous blisibimod (200 mg) or placebo. Patients with renal activity were eligible unless proteinuria exceeded 6 g/24hour or disease severity required escalation of immunosuppressive therapy. Corticosteroid taper was encouraged from Week 8 with the goal to reach ≤7.5 mg prednisone/day. The primary endpoint was the Week 52 SLE Responder Index-6 (SRI-6) in the absence of new/increased immunosuppressives or antimalarials: ≥6-point improvement in SELENA-SLEDAI, no new BILAG 1A or 2B domain scores, and <0.3-point increase in Physician’s Global Assessment.
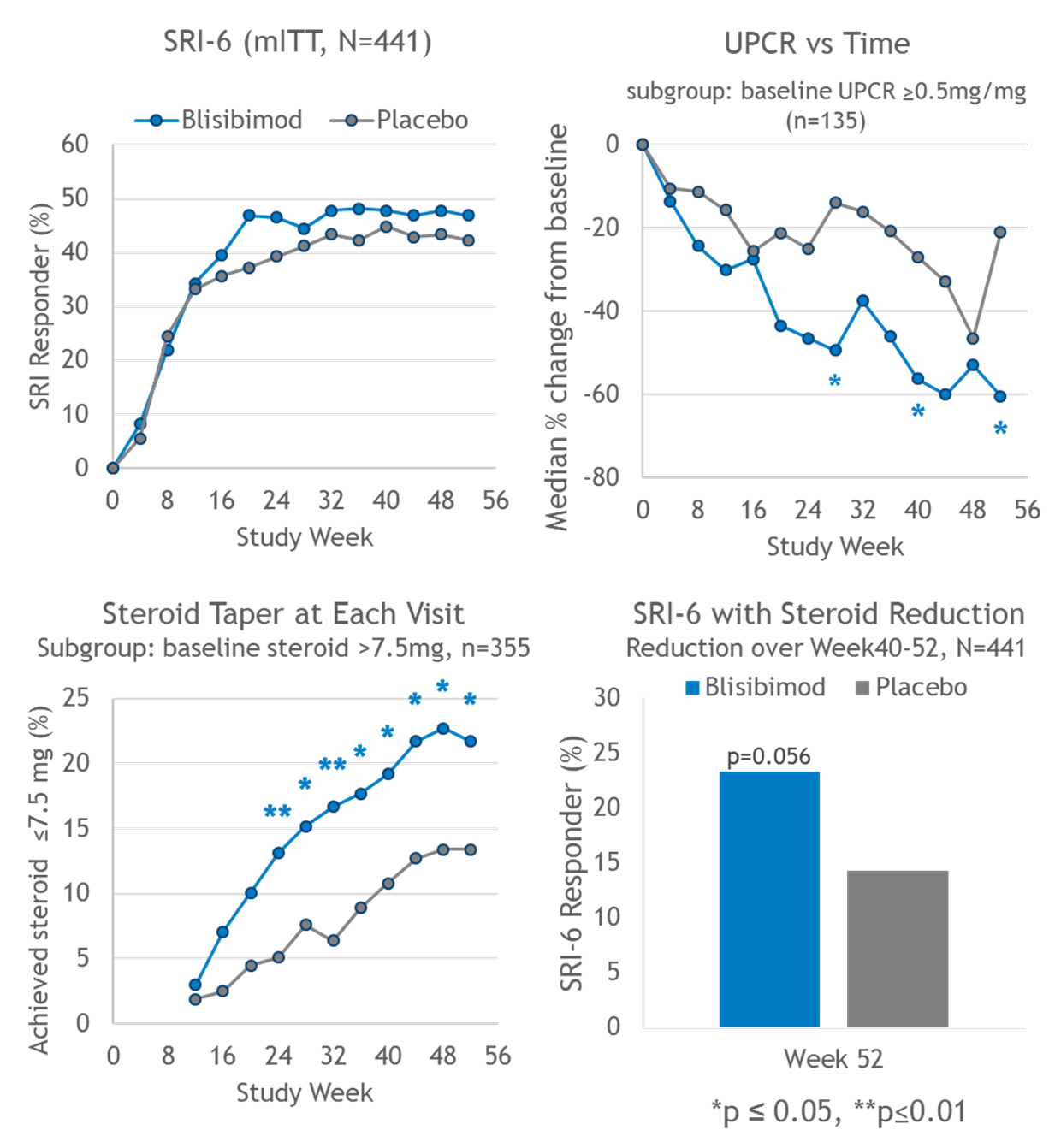
Results: At enrollment, the mean SELENA-SLEDAI score and corticosteroid dose was 13.5±4.2 and 15.6±9.1mg, respectively. The SRI-6 primary endpoint at Week 52 was not met (Figure), and placebo response was greater than reported in previous studies. More blisibimod-treated subjects achieved corticosteroid taper to prednisone ≤ 7.5 mg/day during Weeks 40-52 and better blisibimod effect was observed under the secondary endpoint which, in addition to meeting SRI-6 criteria at Week 52, required corticosteroid dose in Weeks 40-52 to be lower than baseline (Figure). Reductions in anti-dsDNA, significant reductions in peripheral B cell lineages, anti-phospholipid antibodies, and immunoglobulins, and significant increases in complement C3 and C4 were observed with blisibimod.
In a subgroup of subjects with baseline urinary protein:creatinine ratio (UPCR) ≥0.5 mg/mg, greater decreases in UPCR from baseline were observed in the blisibimod arm (Figure). At week 52, significantly more subjects who received blisibimod achieved >50% reduction in UPCR from baseline (59.7 vs 30.8%, p=0.006), and/or UPCR <0.5 (53.2 vs 30.8%, p=0.02).
Adverse events were balanced between treatment arms except injection/application site reactions which were more common with blisibimod. None were serious or severe.
Conclusion: This study did not meet its primary endpoint. Blisibimod treatment was associated with successful steroid reduction, decreased UPCR, and biomarker responses.
References: (1) van Vollenhoven Ann Rheum Dis 2012;71:1343
To cite this abstract in AMA style:
Merrill JT, Martin RS, Shanahan W, Scheinberg M, Kalunian KC, Wofsy D. Phase 3 Trial Results with Blisibimod, a Selective Inhibitor of B-Cell Activating Factor, in Subjects with Moderate-to-Severe Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/phase-3-trial-results-with-blisibimod-a-selective-inhibitor-of-b-cell-activating-factor-in-subjects-with-moderate-to-severe-systemic-lupus-erythematosus/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-3-trial-results-with-blisibimod-a-selective-inhibitor-of-b-cell-activating-factor-in-subjects-with-moderate-to-severe-systemic-lupus-erythematosus/