Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Autologous hematopoietic stem cell (HSC) transplantation is a recommended therapeutic option for selected patients with scleroderma and other autoimmune diseases. HSC transplant requires collection of HSCs via mobilization by G-CSF, which consists of 4-7 days of injections that are associated with significant side effects and potential for severe complications including disease flares (e.g., scleroderma and multiple sclerosis). MGTA-145 is a biologic that activates CXCR2 on neutrophils, and with plerixafor (a CXCR4 antagonist) rapidly mobilizes HSCs in mice and non-human primates. The combination promises to be a same-day, G-CSF-free mobilization regimen.
Methods: This healthy volunteer study consisted of four parts- Part A: single-agent MGTA-145 or placebo; Part B: MGTA-145 or placebo given immediately or 2 hours after plerixafor; Part C: MGTA-145 or placebo given 2 hours after plerixafor on 2 consecutive days; Part D: MGTA-145 given 2 hours after plerixafor, just prior to apheresis cell collection.
Results: Monotherapy of MGTA-145 mobilized CD34+ cells within minutes and peaked within 1-hour post MGTA-145 (median 11 CD34+ cells/µL, a 7-fold increase vs baseline). White blood cells and neutrophils followed a similar pattern. Importantly, markers of neutrophil activation were relatively unchanged (≤2-fold vs baseline, Fig 1).
MGTA-145 combined with plerixafor increased CD34+ cell mobilization, whether given simultaneously or 2h after plerixafor (Fig. 2). Mobilization was highly enriched for CD34+CD90+CD45RA- HSCs. At the 0.03 mg/kg dose with 2h stagger, median peak CD34+ peripheral blood mobilization was ≥40 cells/µL in Part B. On a second consecutive day of dosing, MGTA-145 + plerixafor mobilized HSCs to levels comparable to day 1. Apheresis cell collection yields in Part D showed that sufficient numbers of cells (median 4.3 x 10^6 CD34+ cells/kg) for transplant were collected in a single day. Notably, MGTA-145 + plerixafor mobilized 3-fold higher numbers of CD34+CD90+CD45RA- HSCs and demonstrated >10-fold higher engraftment in NSG mice compared to cells mobilized by G-CSF.
MGTA-145 monotherapy was well tolerated with no significant adverse events (AEs). Grade 1, transient lower back pain that dissipated within minutes was reported. The combination of MGTA-145 with plerixafor was well tolerated, with some subjects experiencing grade 1/2 gastrointestinal AEs commonly observed with plerixafor and one grade 2 back pain with MGTA-145 at 0.075 mg/kg that resolved within minutes.
Conclusion: MGTA-145 was well-tolerated and induced rapid mobilization of significant numbers of HSCs. CD34+ cell mobilization with MGTA-145 + plerixafor was immediate and superior to plerixafor alone. These data suggest that the combination enables the collection of sufficient HSCs for transplant in one day without the need for G-CSF. Further clinical development as a first line mobilization agent is warranted in scleroderma and other autoimmune diseases, as well as in gene therapy and hematologic malignancies.
 Figure 1: MGTA-145 has rapid, on-target neutrophil pharmacodynamics with minimal activation. (A) A single dose of MGTA-145 elicits dose-dependent mobilization of neutrophils into peripheral blood. (B) MGTA-145 monotherapy leads to rapid downregulation of its target receptor, CXCR2, on peripheral blood neutrophils. (C) MGTA-145 monotherapy leads to an increase in the molar ratio of the neutrophil protease, MMP-9, to its inhibitor, TIMP-1, in plasma. (D) MGTA-145 monotherapy elicits only modest changes in peripheral blood neutrophil activation markers (median ≤ 2-fold) after administration. Data represent at least 4 subjects per dose level and are expressed as mean ± SEM (A-C) or median + 10-90 percentile range (D). The shaded region in (A) represents the normal reference range for healthy subjects. The dotted line in (D) represents the anticipated effect of 5 days of G-CSF based on published data (Falanga et al, Blood. 1999).
Figure 1: MGTA-145 has rapid, on-target neutrophil pharmacodynamics with minimal activation. (A) A single dose of MGTA-145 elicits dose-dependent mobilization of neutrophils into peripheral blood. (B) MGTA-145 monotherapy leads to rapid downregulation of its target receptor, CXCR2, on peripheral blood neutrophils. (C) MGTA-145 monotherapy leads to an increase in the molar ratio of the neutrophil protease, MMP-9, to its inhibitor, TIMP-1, in plasma. (D) MGTA-145 monotherapy elicits only modest changes in peripheral blood neutrophil activation markers (median ≤ 2-fold) after administration. Data represent at least 4 subjects per dose level and are expressed as mean ± SEM (A-C) or median + 10-90 percentile range (D). The shaded region in (A) represents the normal reference range for healthy subjects. The dotted line in (D) represents the anticipated effect of 5 days of G-CSF based on published data (Falanga et al, Blood. 1999).
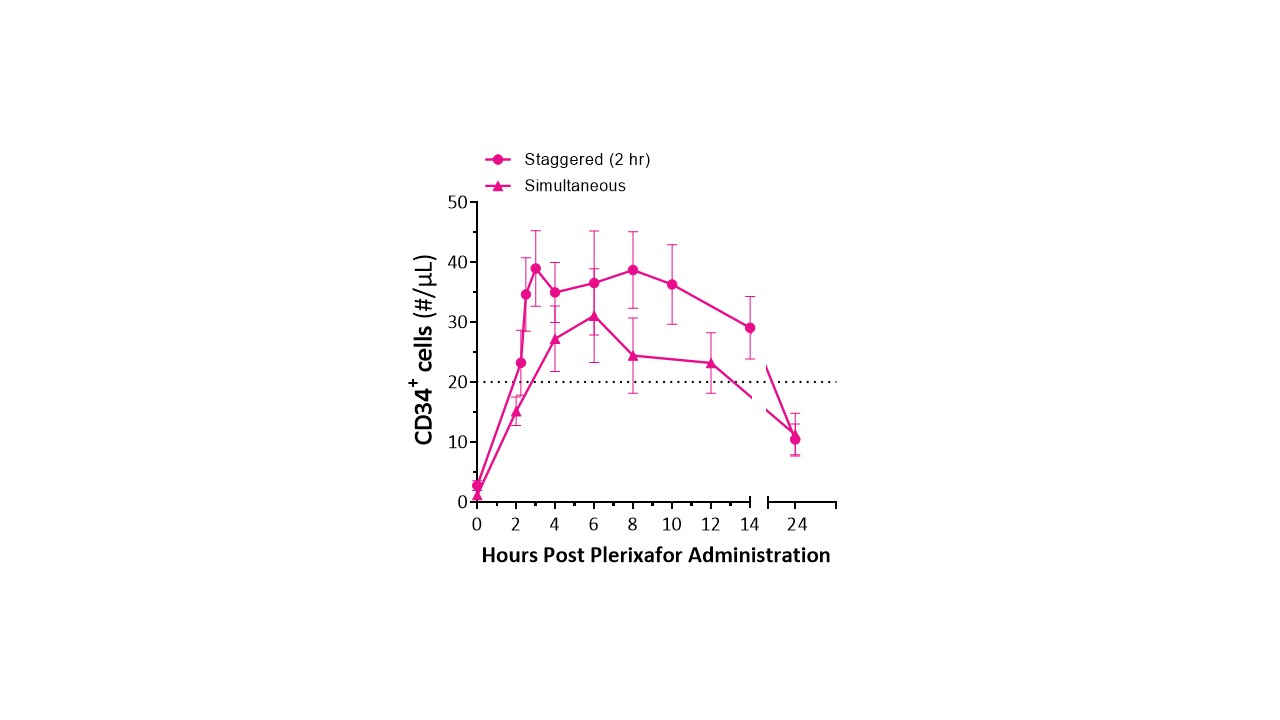 Figure 2. Peripheral blood mobilization after plerixafor + 0.03 mg/kg MGTA-145 in healthy subjects with simultaneous and 2h stagger dosing after plerixafor. Dotted line: previously reported CD34+ counts with plerixafor alone mobilization (Chen et al, Blood Advances. 2018).
Figure 2. Peripheral blood mobilization after plerixafor + 0.03 mg/kg MGTA-145 in healthy subjects with simultaneous and 2h stagger dosing after plerixafor. Dotted line: previously reported CD34+ counts with plerixafor alone mobilization (Chen et al, Blood Advances. 2018).
 Table. Single-day Mobilization and Apheresis Cell Yields in Part D
Table. Single-day Mobilization and Apheresis Cell Yields in Part D
To cite this abstract in AMA style:
DiPersio J, Hoggatt J, Devine S, Scadden D, Howell H, Schmelmer V, Raffel G, Falahee P, Goncalves K, Savage W, Davis J. Phase 1 Clinical Study of MGTA-145 in Combination with Plerixafor Shows Rapid Single-Day Mobilization and Collection of CD34+ Hematopoietic Stem Cells Without G-CSF [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/phase-1-clinical-study-of-mgta-145-in-combination-with-plerixafor-shows-rapid-single-day-mobilization-and-collection-of-cd34-hematopoietic-stem-cells-without-g-csf/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-1-clinical-study-of-mgta-145-in-combination-with-plerixafor-shows-rapid-single-day-mobilization-and-collection-of-cd34-hematopoietic-stem-cells-without-g-csf/
