Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In an open-label trial in adult patients with uncontrolled gout (MIRROR open-label [OL] trial) evaluating pegloticase co-treatment with methotrexate (MTX), 78.6% patients maintained serum uric acid < 6 mg/dL for at least 80% of the time during month 6 [weeks 20, 22, and 24] versus 42% in pegloticase historical monotherapy trials (C0405 and C0406)1. MTX co-treatment can affect the pharmacokinetics (PK) of biologics by attenuating the formation of anti-drug antibodies2. The aim of this analysis was to determine the systemic exposures of pegloticase and methotrexate polyglutamate(s) (MTX-PGs) in uncontrolled gout patients receiving pegloticase and MTX and to evaluate the effect of MTX on the PK and immunogenicity of pegloticase in comparison to historical pegloticase monotherapy trials (C0405 and C0406)3,4.
Methods: In the MIRROR OL trial, MTX (15 mg/week) was given orally 4 weeks prior to the 1st pegloticase dose and continued weekly, in combination with pegloticase 8 mg given intravenously every 2 weeks, for a treatment duration of 52 weeks. Pre-infusion samples were collected to measure MTX-PGs in red blood cells. Pre- and post-infusion blood samples were obtained to measure the peak (Cmax) and trough (Cmin) concentrations of pegloticase at multiple visits. Anti-drug antibody blood samples were collected at multiple visits. The impact of MTX on pegloticase PK was evaluated by comparing pegloticase exposures with MTX from this trial to historical monotherapy data (C0405 and C0406)3,4. The observed pegloticase concentrations with MTX were also overlaid with the 90% prediction interval based on the population PK model from C0405 and C04065.
Results: Pegloticase and MTX-PG levels were determined in fourteen patients. Responders (11/14) were generally associated with higher pegloticase exposures, especially Cmin (Figure 1). Concomitant treatment of MTX resulted in fewer patients with Cmin below quantitation limit (BQL) (5/14 [36%] with MTX vs 63/82 [77%] without MTX), and higher overall Cmin (median: 0.97 µg/ml with MTX vs BQL without MTX); Cmax was slightly higher (median [Q1, Q3]: 2.04 [1.65, 2.68] µg/mL with MTX vs 1.51 [BQL, 2.48] µg/mL without MTX). Pegloticase co-treatment with MTX resulted in more concentrations above the predicted median value of pegloticase, compared to monotherapy (Figure 1,2). This may be due to the effect of MTX in reducing the immunogenicity of pegloticase. Concentrations of MTX-PGs were maintained during the treatment course, suggesting compliance of MTX administration. There was no apparent relationship between response and MTX-PGs exposures.
Conclusion: Pegloticase 8 mg IV every 2 weeks co-treatment with MTX 15 mg weekly resulted in fewer patients with pegloticase Cmin BQL, and higher overall Cmin compared to pegloticase monotherapy and was associated with an improved response rate for pegloticase in patients with uncontrolled gout.
References:
1. Botson J., et al. Ann Rheum Dis; 2020, 79(S1).
2. Goss S. L., et al. Clin Ther; 2018, 40(2).
3. Lipsky P. E., et al. Arthritis Res Ther; 2014, 16(2).
4. Sundy J. S., et al. JAMA; 2011, 306(7).
5. Yue C. S., et al. ASCPT, Atlanta, 2010.
 Figure 1. Comparison of pegloticase exposures (Cmax and Cmin) in methotrexate co-treatment (MIRROR OL) and monotherapy trials (C0405 and C0406)
Figure 1. Comparison of pegloticase exposures (Cmax and Cmin) in methotrexate co-treatment (MIRROR OL) and monotherapy trials (C0405 and C0406)
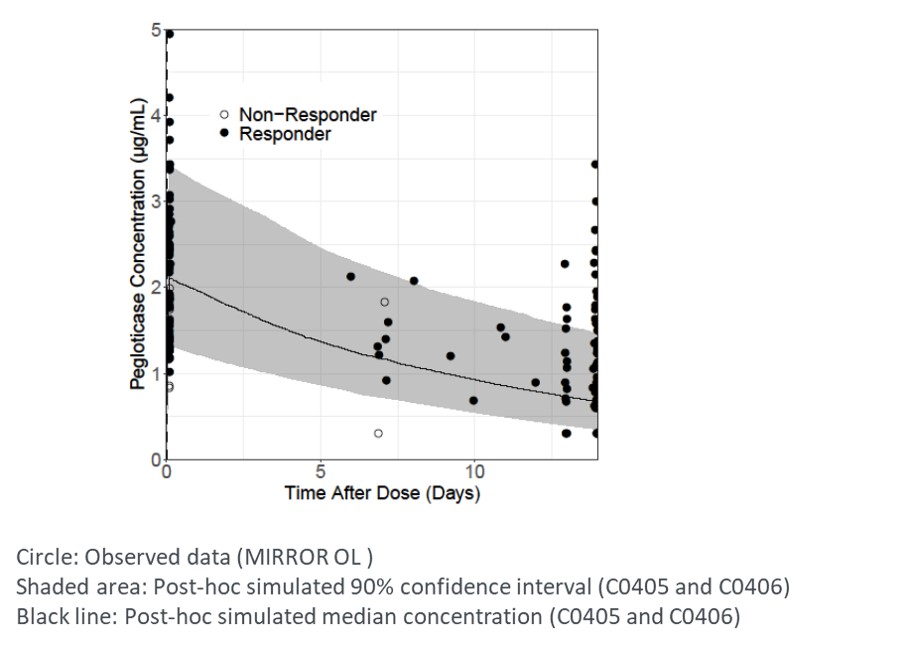 Figure 2. Comparison of observed pegloticase concentrations in MIRROR OL and stimulated PK profile of pegloticase as monotherapy
Figure 2. Comparison of observed pegloticase concentrations in MIRROR OL and stimulated PK profile of pegloticase as monotherapy
To cite this abstract in AMA style:
Song Y, Xin Y, Weinblatt M, Chamberlain J, Obermeyer K, Zhao L, Canavan C, Peloso P, Ramanathan S. Pharmacokinetics of Pegloticase and Methotrexate Polyglutamate(s) in Patients with Uncontrolled Gout Receiving Pegloticase and Co-treatment of Methotrexate [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pharmacokinetics-of-pegloticase-and-methotrexate-polyglutamates-in-patients-with-uncontrolled-gout-receiving-pegloticase-and-co-treatment-of-methotrexate/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-of-pegloticase-and-methotrexate-polyglutamates-in-patients-with-uncontrolled-gout-receiving-pegloticase-and-co-treatment-of-methotrexate/
