Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: MM-II is a first-in-class investigational treatment for knee OA pain that consists of a one-time IA injection of empty liposomes comprised of phospholipids dipalmitoylphosphatidylcholine (DPPC) and 1,2-dimyristoyl-phosphatidylcholine (DMPC). DPPC is a major surface-activated phospholipid that is a part of the cartilage boundary lubrication system. In a phase 2b, dose-finding clinical trial, a single injection of 3 mL MM-II provided sustained reduction in knee pain (measured by weekly average of daily knee pain score as well as WOMAC pain score) compared to placebo, starting from 6 weeks after injection and sustained through 26 weeks. MM-II was safe and well tolerated, with no serious treatment-emergent adverse events, and safety profiles were consistent between placebo and MM-II. Here, we report the pharmacokinetic (PK) profiles of DMPC and DPPC from the trial following a single IA injection of MM-II compared to placebo.
Methods: In a phase 2b, randomized, double-blind, dose-ranging, placebo-controlled trial (NCT04506463), 397 participants with chronic knee OA were randomized 3:3:3:1:3:1 to receive 1, 3, or 6 mL MM-II or 1, 3, or 6 mL placebo through a single IA injection. All participants met the ACR Clinical and Radiological Criteria for chronic knee OA. Blood samples for PK measurements were taken from participants at a subset of study centers, at the following time points: pre-injection (at baseline), and post-injection at 4 hours, 2 days (48 hours), and 7 days (168 hours). Plasma DPPC and DMPC concentrations (ng/mL) were quantified at each time point and for each study arm using liquid chromatography tandem mass spectrometry. Concentrations below the lower limit of quantification were analyzed as 0 before the first quantifiable concentration and as missing after the first quantifiable concentration. Analyses were based on the modified PK analysis set, which included participants who received the single dose of IA injection, had at least 1 quantifiable post-dose concentration, and did not have major protocol deviations. PK parameters were summarized using descriptive statistics based on baseline-adjusted values.
Results: The modified PK analysis set included 78 participants. Quantifiable levels of both DMPC and DPPC were found in pre-dose samples from MM-II and I placebo controls. There were no considerable changes in plasma DMPC and DPPC concentrations from pre-dose to post-dose time points (Figure 1). Furthermore, the concentrations of DMPC and DPPC appeared to be comparable between MM-II and corresponding placebo groups at all time points. These data suggest there was no increase in measurable DMPC or DPPC levels after administration of MM-II within the time points analyzed.
Conclusion: Based on these results, treatment of OA knee pain with a single IA injection of MM-II did not appear to increase circulating DMPC and DPPC levels compared with placebo within the time points analyzed. Further studies with additional sampling are necessary to enhance interpretation due to limited sampling time points and inherent variability in PK parameters.
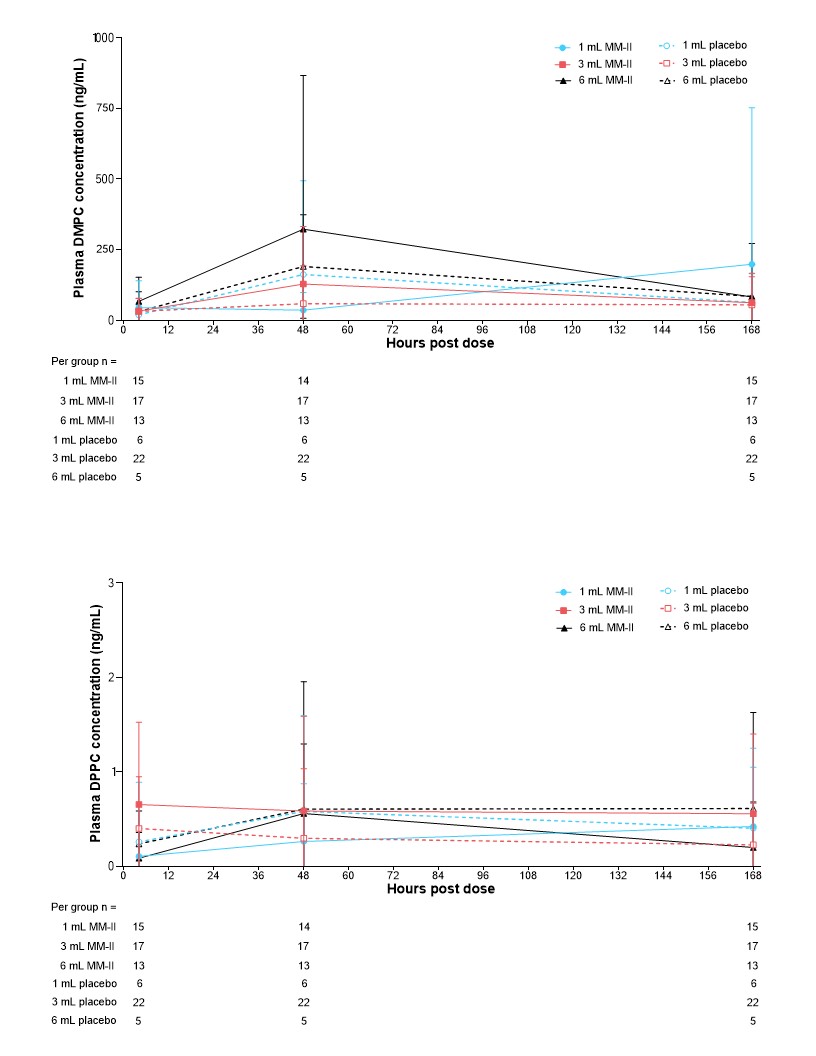 Figure 1. Mean ± SD baseline-adjusted DMPC and DPPC concentrations up to 168 h after intra-articular injection of MM-II or placebo.
Figure 1. Mean ± SD baseline-adjusted DMPC and DPPC concentrations up to 168 h after intra-articular injection of MM-II or placebo.
DMPC, 1,2-dimyristoyl-phosphatidylcholine; DPPC, dipalmitoylphosphatidylcholine; SD, standard deviation.
To cite this abstract in AMA style:
Schnitzer T, Chevalier X, Rovsing H, Lau E, Boll S, Brahmachari B, Chou R, Joshi T, Wechsler R, Weiner S, Kothekar M, Bihlet A, Conaghan P. Pharmacokinetic Profile of MM-II Following a Single Intra-Articular Injection in Patients with Knee Osteoarthritis in a Phase 2b Randomized, Controlled, Dose-Ranging Trial [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetic-profile-of-mm-ii-following-a-single-intra-articular-injection-in-patients-with-knee-osteoarthritis-in-a-phase-2b-randomized-controlled-dose-ranging-trial/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetic-profile-of-mm-ii-following-a-single-intra-articular-injection-in-patients-with-knee-osteoarthritis-in-a-phase-2b-randomized-controlled-dose-ranging-trial/
