Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Adalimumab, a recombinant fully human monoclonal immunoglobulin G1 antibody, is a biologic directed against tumor necrosis factor-alpha indicated for use in a range of autoimmune diseases. MSB11022, an adalimumab biosimilar, has demonstrated equivalent pharmacokinetics (PK) and efficacy as well as comparable safety and immunogenicity to the EU-reference adalimumab,1–3 and was approved in the EU in a citrate buffer formulation in 2019.4 An acetate buffer formulation of MSB11022 (MSB11022-acetate) has been developed for introduction in the US. This study compared the PK, safety, tolerability, and immunogenicity profiles of MSB11022-acetate versus the US-reference adalimumab (US-RP) and MSB11022. Here we present data on the PK equivalence of MSB11022-acetate and US-RP. Results for 3 treatment arms will be presented at a later stage.
Methods: In this randomized, double-blind, 3-arm parallel-group study, healthy subjects were randomized (1:1:1) to receive a single 40 mg subcutaneous dose of MSB11022-actetate, MSB11022, or US-RP via a pre-filled syringe on Day 1. Randomization was stratified by body weight category. Blood samples for PK and immunogenicity were taken pre-dose and at scheduled intervals up to Day 71 post-dose. The primary endpoint PK parameters were area under the concentration–time curve from time 0 to infinity, maximum concentration, and area under the concentration–time curve from time 0 to the last quantifiable concentration. Primary parameters were log-transformed and compared between treatment arms using an analysis of variance model with treatment as a fixed effect and randomization stratification as a cofactor. Safety and immunogenicity endpoints were collected throughout the study and summarized using descriptive statistics.
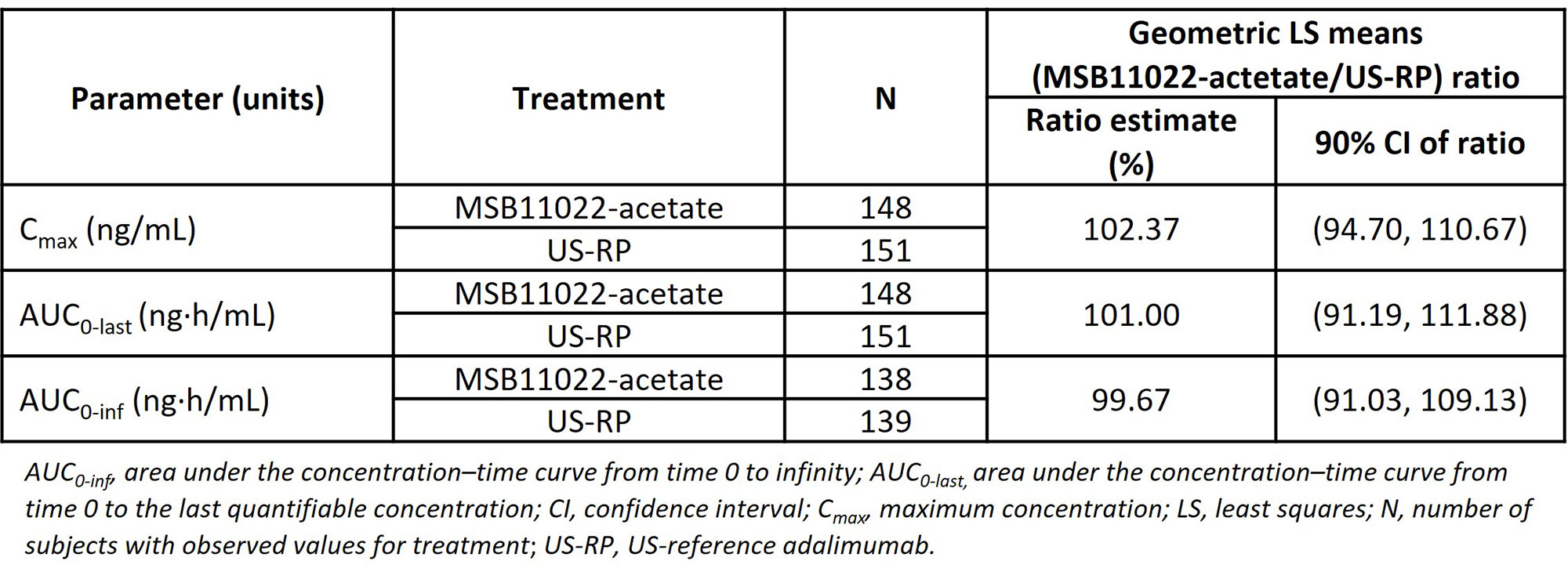
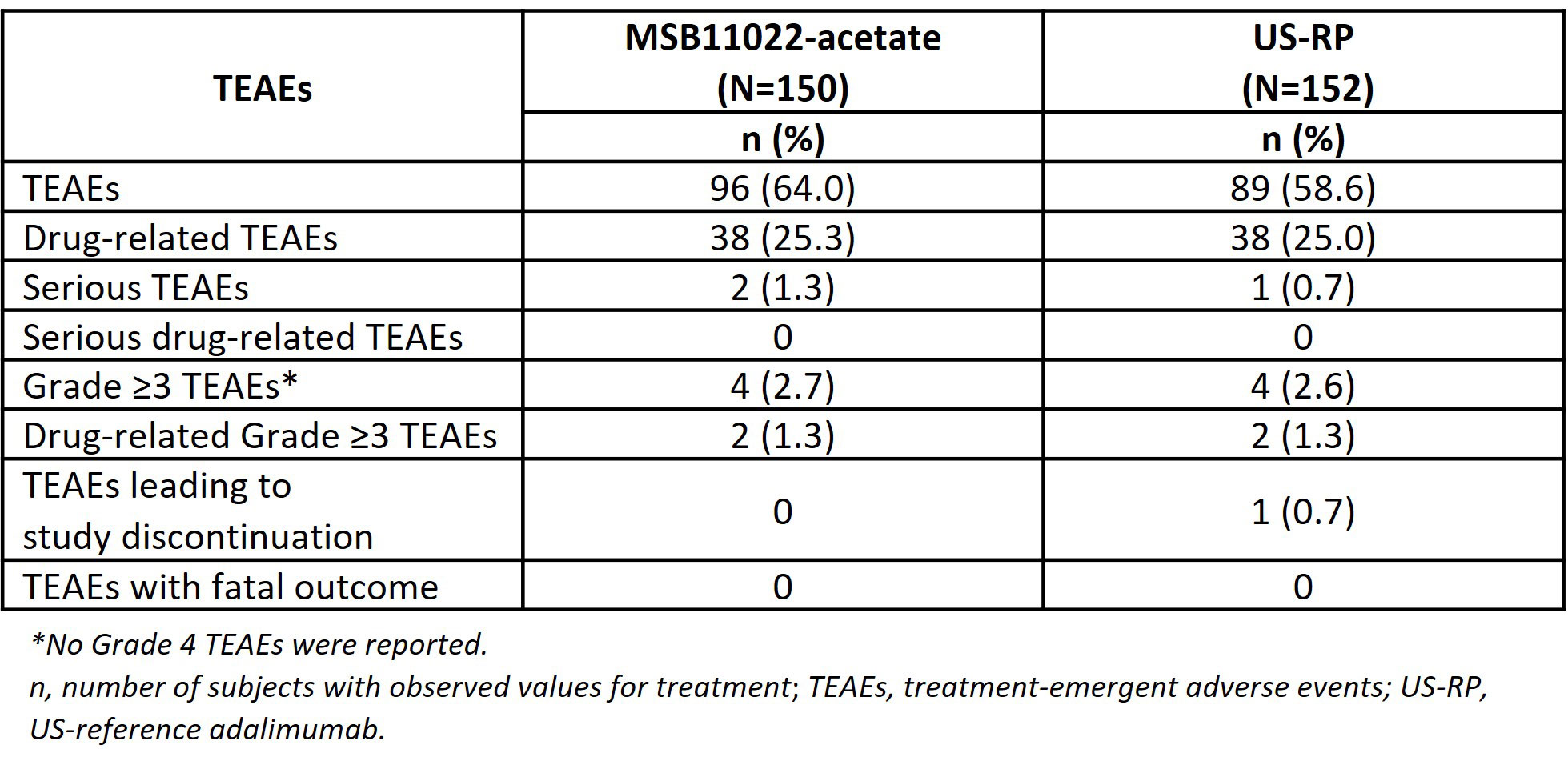
Results: Of the 459 subjects randomized, 452 received a single dose of study drug: MSB11022-acetate, n=150; MSB11022, n=150; US-RP, n=152. The primary PK analysis demonstrated equivalence between MSB11022-actetate and US-RP; the 90% confidence intervals for the geometric least squares mean ratios were completely within the predefined equivalence margins of 80.00% to 125.00% for all primary PK parameters (Table 1). MSB11022-acetate and US-RP had comparable safety profiles, no serious adverse events were considered related to the study drug, and there were no reported deaths (Table 2). The immunogenicity profile of MSB11022-acetate was similar to US-RP, with comparable incidence and titer of anti-drug antibodies and incidence of neutralizing antibodies at all time points.
Conclusion: PK equivalence of MSB11022-acetate and US-RP was demonstrated with comparable immunogenicity, safety, and tolerability profiles. This study adds to the totality of evidence in support of MSB11022-acetate as a proposed adalimumab biosimilar.
References
1. Hyland E, et al. Br J Clin Pharmacol 2016;82:983–93.
2. Hercogová J, et al. Br J Dermatol 2020;182:316–26.
3. Edwards CJ, et al. Clin Rheumatol 2019;38:3381–90.
4. EMC. Idacio SmPC. Last updated Jan 11, 2022. https://www.medicines.org.uk/emc/product/11307/smpc#gref
To cite this abstract in AMA style:
Dryja A, Buccarello A, Gaillard I, Monnet J. Pharmacokinetic Evaluation of a Proposed Adalimumab Biosimilar MSB11022 versus the US-Licensed Reference Product: Results of a Randomized, Double-Blind, 3-Arm Parallel-Group, Single-Dose Trial in Healthy Subjects [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetic-evaluation-of-a-proposed-adalimumab-biosimilar-msb11022-versus-the-us-licensed-reference-product-results-of-a-randomized-double-blind-3-arm-parallel-group-single-dose-trial-in-heal/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetic-evaluation-of-a-proposed-adalimumab-biosimilar-msb11022-versus-the-us-licensed-reference-product-results-of-a-randomized-double-blind-3-arm-parallel-group-single-dose-trial-in-heal/