Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: There are limited data regarding persistency of TNF inhibitor (TNFi) therapies in patients with ankylosing spondylitis (AS) treated in real-world clinical practice. The objective of this analysis was to evaluate time to discontinuation of a TNFi therapy in patients with AS in the US-based Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry.
Methods: This analysis included all patients with AS aged ≥ 18 years enrolled in the Corrona PsA/SpA registry between April 2013 and January 2015 who were receiving or initiated a TNFi therapy at the time of registry enrollment (index visit) and had ≥ 1 follow-up visit. Patient demographics, clinical characteristics, patient-reported outcomes (PROs) and treatment history at baseline were summarized descriptively. Median (95% CI) time from enrollment to discontinuation of the index TNFi therapy was estimated using Kaplan-Meier analysis.
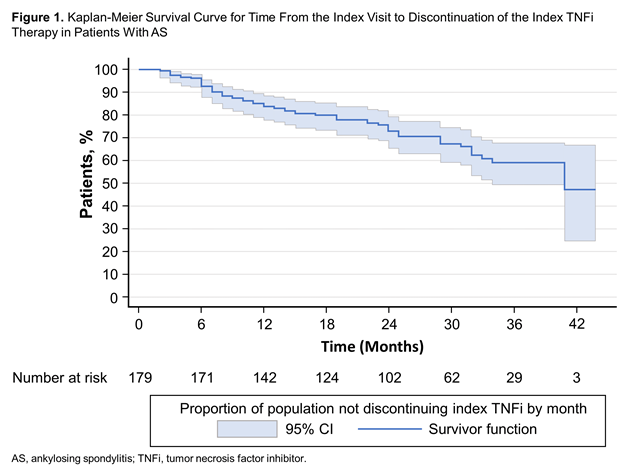
Results: Of the 179 patients included in the analysis, 30.3% were female, 91.8% were white, mean (SD) age was 47.8 (14.0) years and mean (SD) body mass index was 29.3 (6.9) kg/m2 (Table 1). The mean (SD) disease duration was 18.3 (12.7) years, and 89.9% of patients had received prior biologic therapy. At baseline, the mean (SD) Bath Ankylosing Spondylitis Disease Activity Index and Bath Ankylosing Spondylitis Functional Index scores were 3.9 (2.5) and 3.2 (2.8), respectively, and patients reported mean (SD) pain and fatigue scores of 38.1 (30.2) and 45.5 (29.4), respectively; additional disease activity measures and PROs are described in Table 1. A total of 57 patients (31.8%) discontinued their index TNFi therapy during follow-up. The median (95% CI) time from enrollment to TNFi discontinuation was 41 (34 to not estimable) months (Figure 1).
Conclusion: Approximately 30% of patients with AS who received a TNFi therapy at time of enrollment in the Corrona PsA/SpA registry and had ≥ 1 follow-up visit discontinued their index TNFi therapy, with a median time to discontinuation of 41 months. These results provide insight into the persistency of TNFi therapy in US patients with AS in a real-world setting.
To cite this abstract in AMA style:
Mease PJ, van der Heijde D, Karki C, Liu M, Park Y, Greenberg JD. Persistency of Tumor Necrosis Factor Inhibitor Therapy in US Patients with Ankylosing Spondylitis: Data from the Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/persistency-of-tumor-necrosis-factor-inhibitor-therapy-in-us-patients-with-ankylosing-spondylitis-data-from-the-corrona-psoriatic-arthritisspondyloarthritis-psaspa-registry/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistency-of-tumor-necrosis-factor-inhibitor-therapy-in-us-patients-with-ankylosing-spondylitis-data-from-the-corrona-psoriatic-arthritisspondyloarthritis-psaspa-registry/