Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: AM
stiffness is a common yet under-appreciated
symptom in RA. The
longitudinal impact of AM stiffness has not been previously investigated. This
study evaluated
persistency of AM stiffness over
time and prevalence of improvement based
on decreases in stiffness duration following
initiation of new
DMARD therapy in the Corrona RA
Registry.
Methods: Adult
patients enrolled as of December 31, 2014 who initiated new biologic or synthetic DMARDs and maintained treatment for ≥90 days were analyzed and followed until last visit. The proportion and frequency of
patients who reported none or decreased duration of AM stiffness were compared with those with persistent or increased
duration at last visit. Among patients with AM stiffness at treatment
initiation, baseline characteristics and categorical improvement in stiffness duration
were evaluated. Patients reporting <1hr of AM stiffness were considered
improved if they had no stiffness; those reporting 1-<2hrs were improved if <0.5 hours and those with 2-<3 and ≥3hrs improved if <1hr by last visit.
Results:
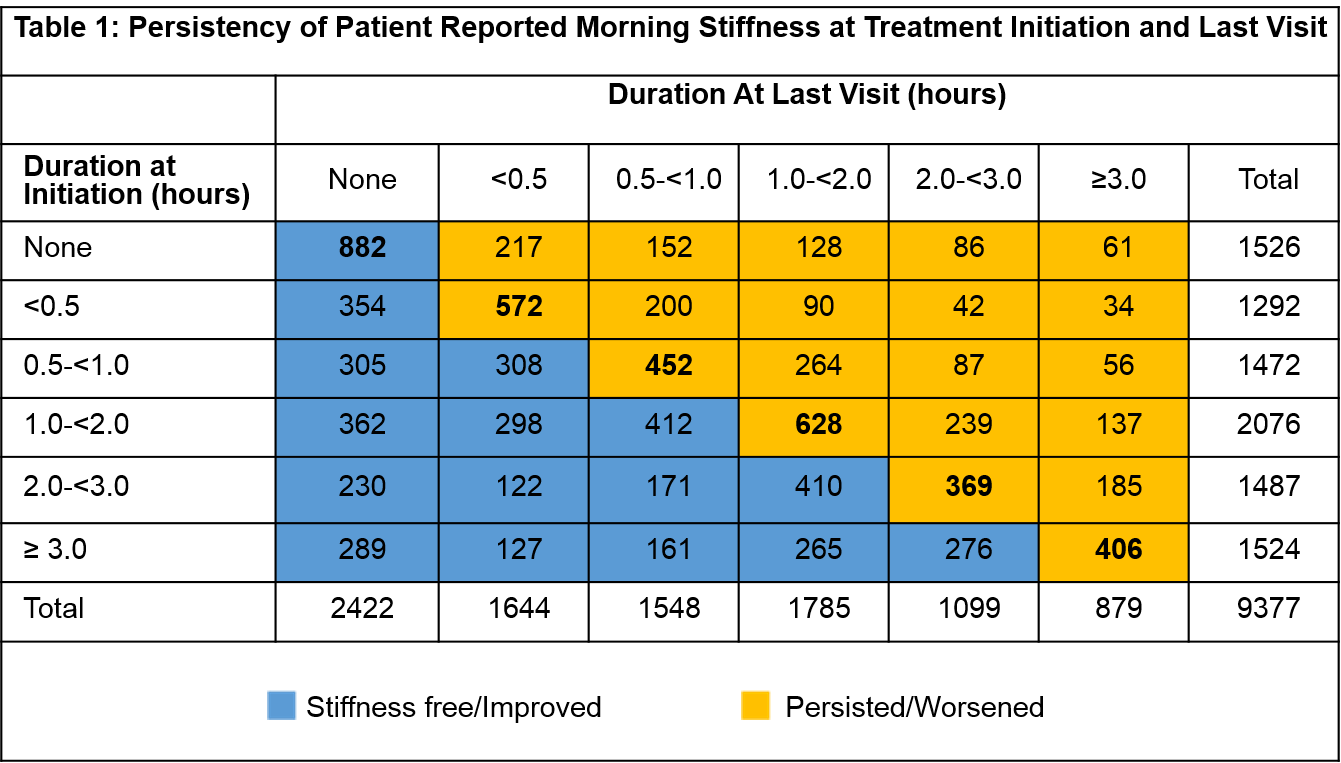
Of 9377 total patients, 4972 (53%) continued
stiffness free or reported decreased
duration and 4405 (47%) had persistent or worsened duration of stiffness at
last visit (Table 1). 644 (42.2%) with no stiffness at treatment initiation reported stiffness by last visit. Among 7851 with AM stiffness
at treatment initiation, a large majority were biologic
DMARD naïve
(56.5%) and initiated combination therapy
(56.4%) (Table 2). When categorical improvement in stiffness
duration criteria were analyzed, 5432
(69.2%) among patients reporting AM stiffness at
treatment initiation reported no improvement at
last visit.
Conclusion: In this
analysis, longitudinal and categorical
change data demonstrate that AM
stiffness is a recurring symptom which persists as an unmet need
in a large majority of RA patients despite initiation of new DMARD therapy.
Further research is needed to evaluate clinically meaningful metrics from the
patients‘
perspective for improvement in AM stiffness duration. Clinical factors associated with improved responses should be
evaluated to address persistency of AM stiffness that continues to affect a
large number of RA patients.
To cite this abstract in AMA style:
Strand V, Holt RJ, Litman HJ, Kent JD, Pashova H, Nguyen JT, Etzel CJ. Persistency of Patient Reported Morning (AM) Stiffness in a Large US Registry Cohort of Rheumatoid Arthritis (RA) Patients Initiating New DMARD Therapy [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/persistency-of-patient-reported-morning-am-stiffness-in-a-large-us-registry-cohort-of-rheumatoid-arthritis-ra-patients-initiating-new-dmard-therapy/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistency-of-patient-reported-morning-am-stiffness-in-a-large-us-registry-cohort-of-rheumatoid-arthritis-ra-patients-initiating-new-dmard-therapy/