Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Understanding persistence of biologic therapies and factors associated with discontinuation can help inform treatment decisions for patients with rheumatoid arthritis (RA). We sought to evaluate the persistence of tocilizumab (TCZ) therapy and identify factors associated with its discontinuation among US patients with RA in routine clinical practice.
Methods: Eligible participants were TCZ-naïve patients enrolled in the Corrona RA registry who initiated TCZ after January 1, 2010 and had ≥ 1 follow-up visit. Persistence of therapy was defined as maintaining continuous TCZ treatment with no interruptions; patients were considered no longer persistent upon the first discontinuation of TCZ. Persistence was calculated using Kaplan-Meier survival analysis for the overall population; secondary analyses evaluated persistence excluding patients who stopped TCZ with no reported reason for discontinuation (patients with non-medical reasons for discontinuation [eg, insurance] were censored) and in only those patients who initiated intravenous (IV) TCZ. Cox proportional hazards modeling was used to identify factors associated with persistence.
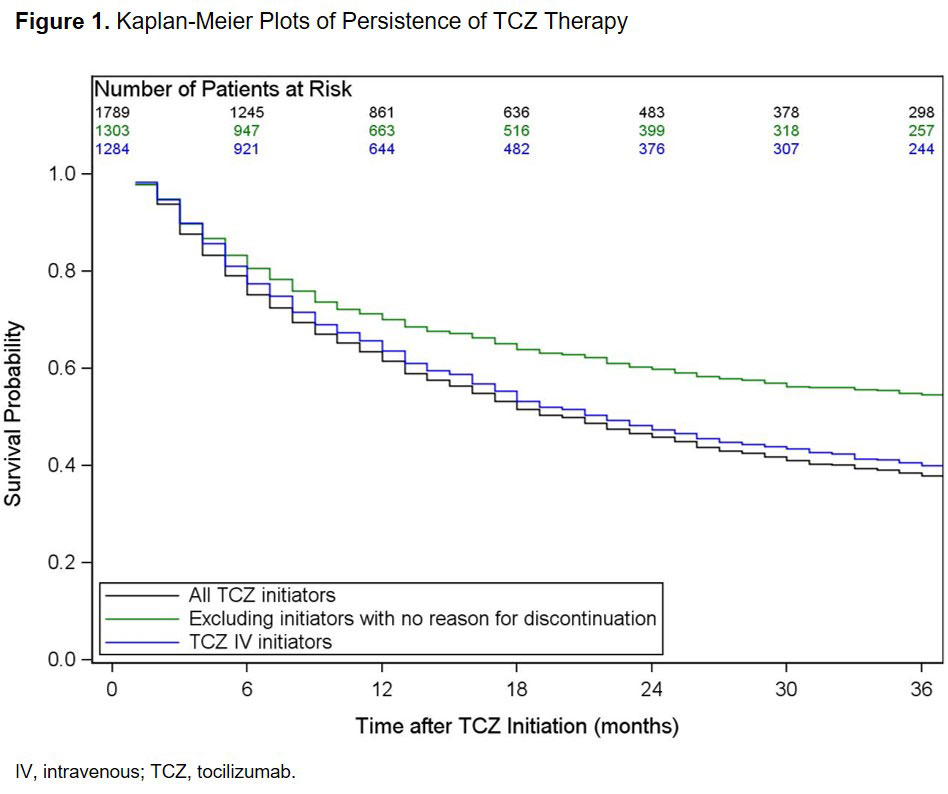
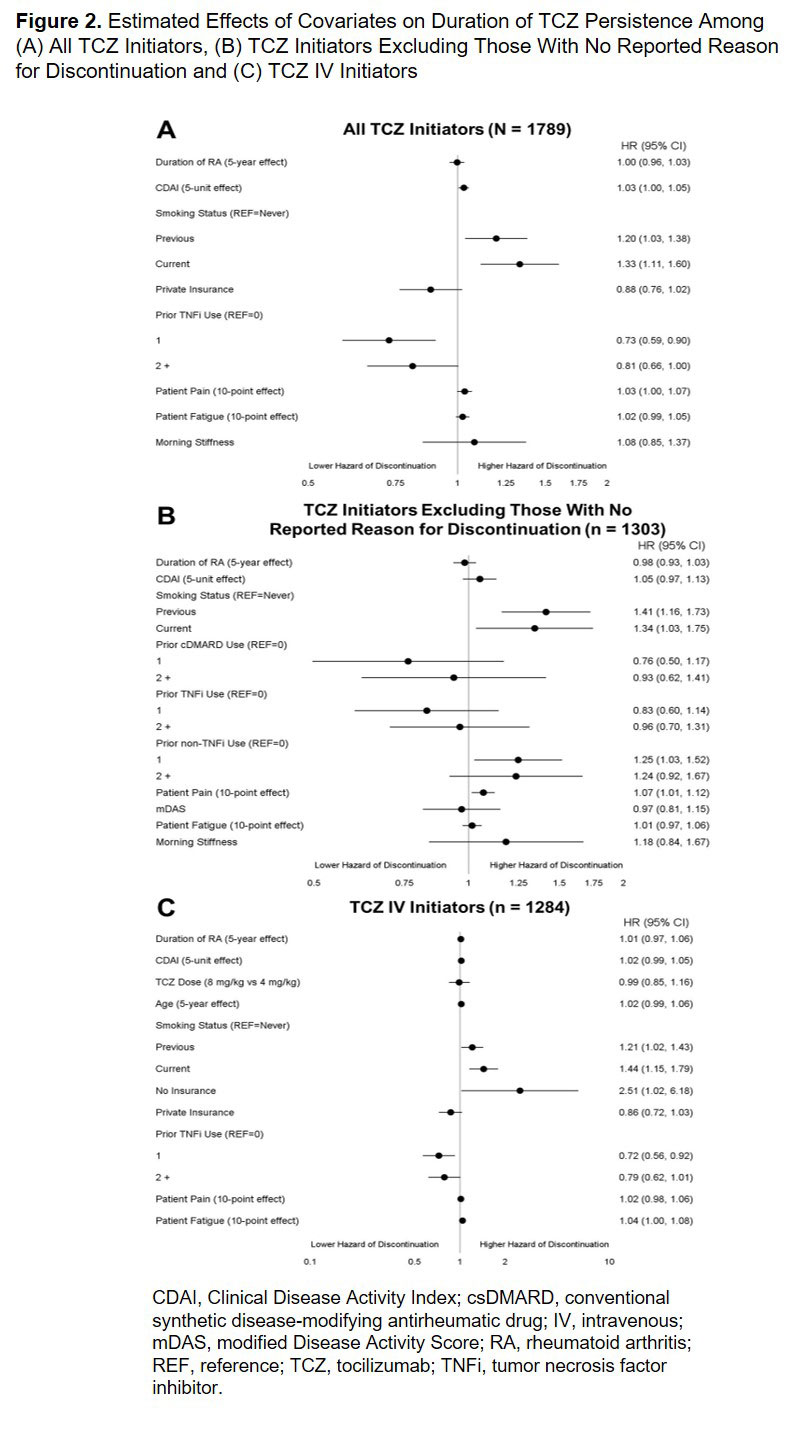
Results: A total of 1789 TCZ initiators were included. Patient characteristics at the time of TCZ initiation are summarized in Table 1. Most patients (93.4%) had prior biologic use and 67.4% had received ≥ 2 prior biologics (Table 1). Overall, 28.8% initiated TCZ as monotherapy (Table 1). Among all TCZ initiators, the median (95% CI) duration of persistence was 20 (18 to 22) months (Figure 1). Factors associated with an increased hazard of TCZ discontinuation included smoking and higher baseline CDAI, whereas prior tumor necrosis factor inhibitor (TNFi) use was associated with a reduced hazard (Figure 2A). After excluding patients with no reported reason for discontinuation (remaining n = 1303), the median (95% CI) duration of persistence was 46 (38 to 55) months (Figure 1); smoking, use of 1 prior non-TNFi and higher baseline patient pain score were associated with an increased hazard of discontinuation (Figure 2B). Among the 1284 patients who initiated TCZ IV, median (95% CI) duration of persistence was 22 (19 to 25) months (Figure 1); smoking, lack of insurance and higher baseline patient fatigue score were associated with a increased hazard of discontinuation, whereas use of 1 prior TNFi was associated with a decreased hazard (Figure 2C).
Conclusion: In this real-world population of US patients with RA, TCZ was most frequently initiated after an inadequate response to ≥ 2 biologics. Overall median duration of persistence was approximately 20 months and was higher (46 months) when patients with no reported reason for TCZ discontinuation were excluded. As expected, factors indicative of higher baseline disease activity were associated with shorter persistence.
Acknowledgements: Support for third-party writing assistance, furnished by Health Interactions, Inc, was provided by Genentech, Inc.
To cite this abstract in AMA style:
Pappas D, Blachley T, Best J, Zlotnick S, Emeanuru K, Kremer J. Persistence of Tocilizumab Therapy Among Patients with Rheumatoid Arthritis: Data from the US-Based Corrona Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/persistence-of-tocilizumab-therapy-among-patients-with-rheumatoid-arthritis-data-from-the-us-based-corrona-rheumatoid-arthritis-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-of-tocilizumab-therapy-among-patients-with-rheumatoid-arthritis-data-from-the-us-based-corrona-rheumatoid-arthritis-registry/