Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: To describe the real-world treatment patterns, response and persistence in rheumatoid arthritis (RA) patients (pts) treated with upadacitinib (UPA).
Methods: This retrospective, non-interventional, multicenter cohort study used data from the OPAL dataset, derived from electronic medical records. Included pts were aged between 18 and 94 years, had a clinical diagnosis of RA and initiated UPA for the first time between May 2020 and March 2023. Pts were followed up to June 2023. Primary objective: Persistence (Kaplan-Meier methods) in the overall population, by line of therapy and as monotherapy and combination therapy. Secondary objectives included describing treatment patterns and clinical effectiveness, measured by DAS28CRP(3) (as observed). To descriptively compare outcomes in pts initiated at index with UPA monotherapy and UPA in combination with csDMARDs, pts were propensity score matched using age, sex, line of therapy, DAS28CRP(3) category, index year.
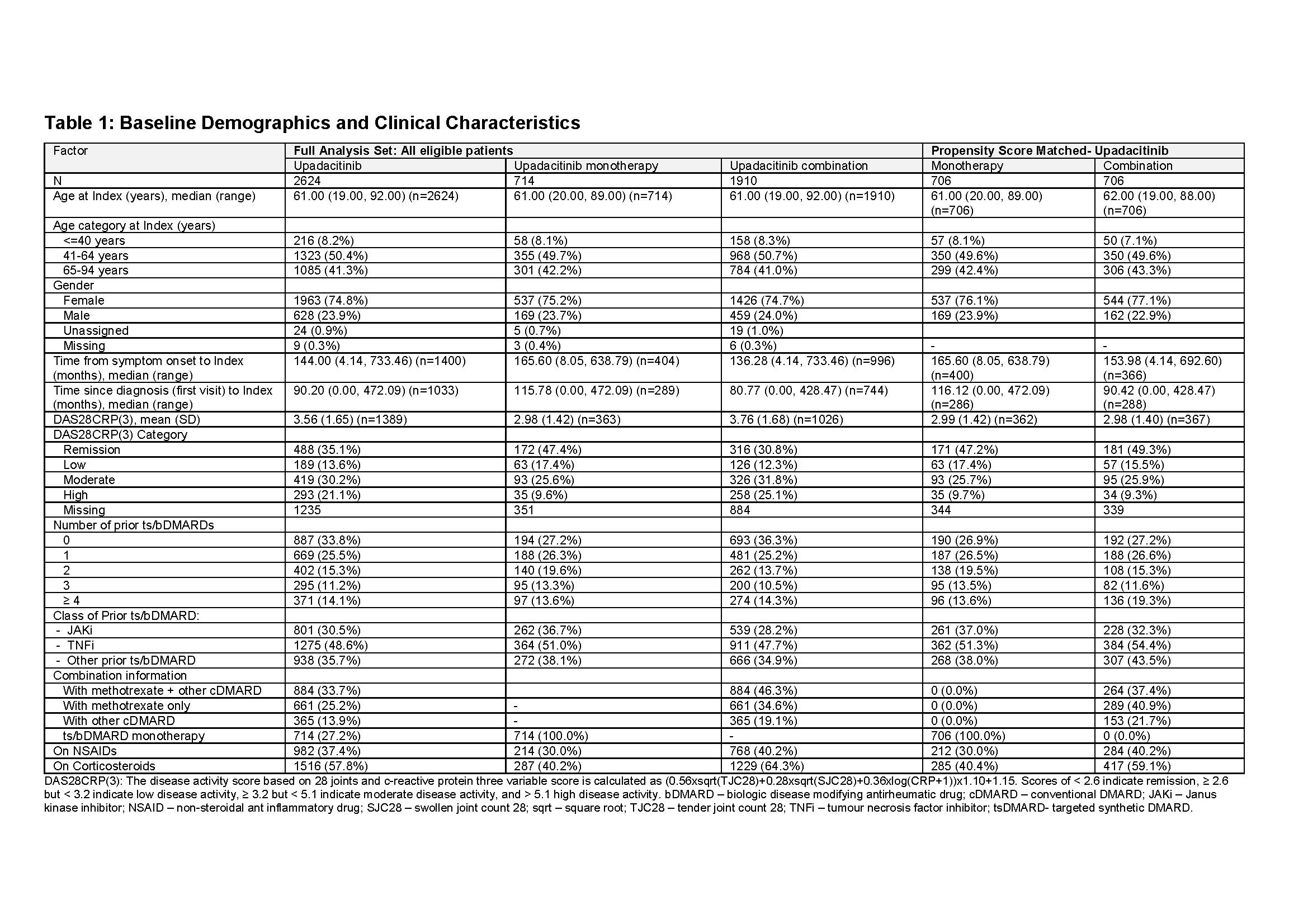
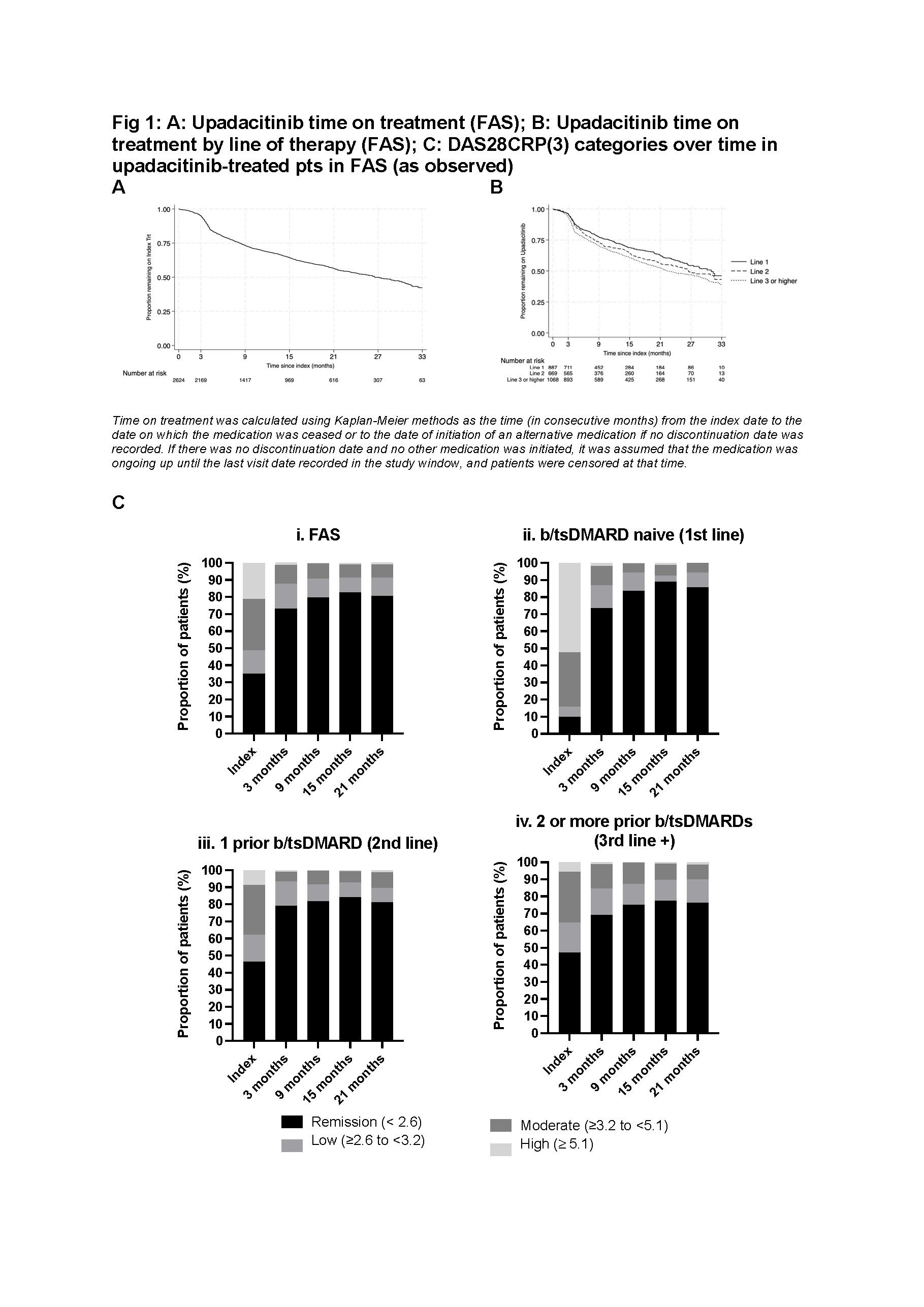
Results: Full Analysis Set (FAS) included 2,624 UPA pts (Table 1). At index, 27% of pts initiated UPA as monotherapy and 73% as combination therapy. 75% of UPA pts were female. Median age of pts was 61 years, with 41% aged ≥ 65 years. 66% were previously treated with b/tsDMARDs and, at index, 37% and 58% of pts were prescribed concomitant NSAID and corticosteroids, respectively. UPA pts had median follow-up of 18.2 months (95% CI 17.1 – 19.1 months), with median time on treatment of 26.6 months (95% CI 24.4 – 29.9 months). UPA persistence rates at 15 and 21 months were 64% (95% CI 62%-66%) and 56.5% (95% CI 54%-59%), respectively. UPA median time on treatment was greatest when used in 1st line (31.0 months in 1st line, 26.5 in 2nd line; 22.4 in ≥3rd line). Persistence rates at 21 months: 63% 1st line, 56% 2nd line, 52% ≥ 3rd line (Fig 1). In 2nd line UPA pts, median time on treatment was similar regardless of prior treatment being JAKi (median 25.3 [95%CI 16.1 – not reached] months) or TNFi (median 27.78 [95% CI 23.2 – 35.4] months). At index, 35% of UPA pts were in DAS28CRP(3) remission (10% in 1st line), increasing to 73% at 3 months (≥ 69% regardless of line of therapy) (Fig 1). The proportion of pts receiving UPA as monotherapy increased to ≥ 33% from 9 months post index.
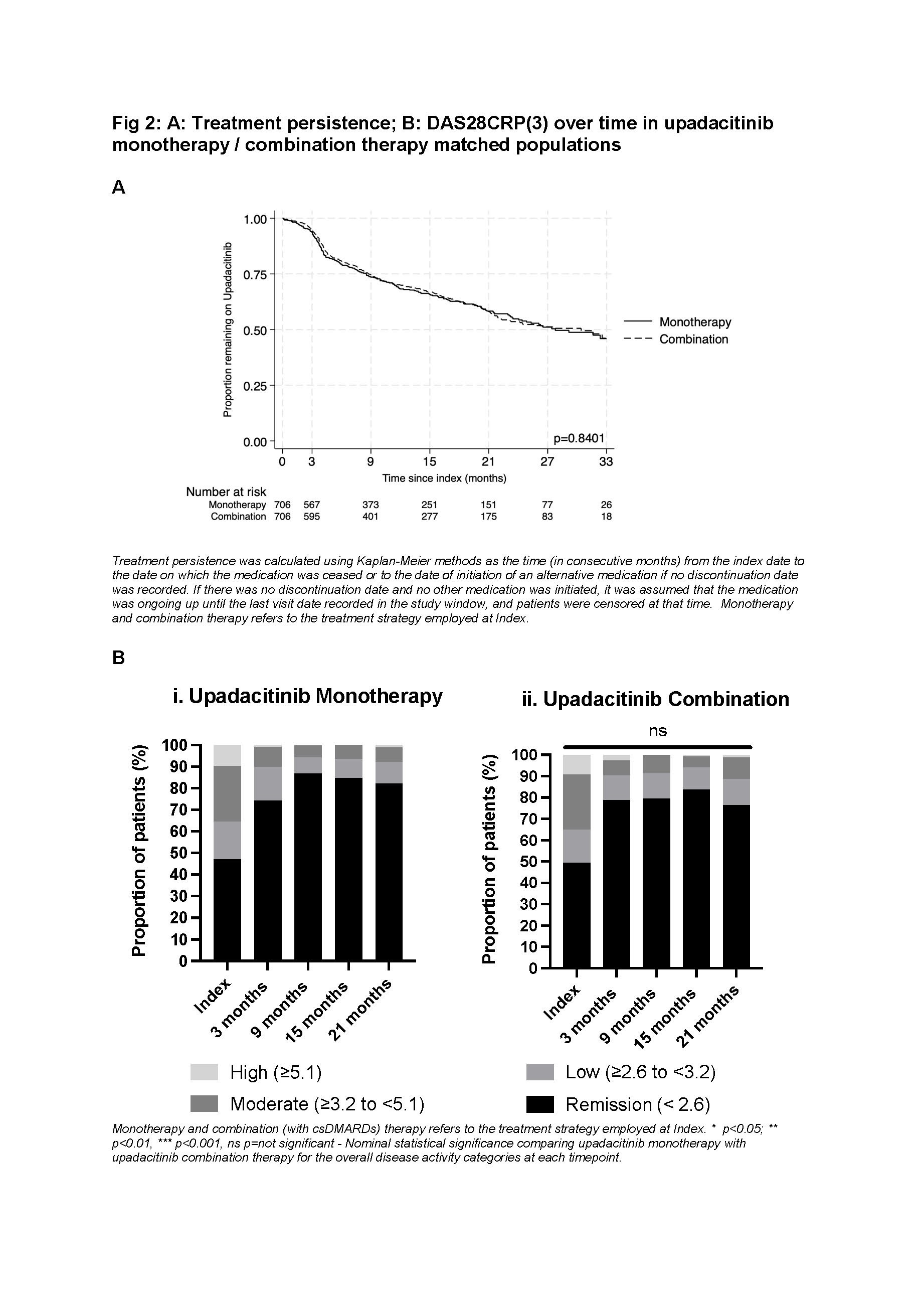
The propensity score matched UPA monotherapy and UPA combination cohorts, included 706 pts each and were generally well matched (Table 1). Median time on treatment was similar for pts receiving at index UPA monotherapy or UPA combination (27.8 months [95% CI 23.5 to 33.4 months] and 30.4 [95% CI 22.1 to 35.3 months], respectively; p=0.84). At index, almost half of UPA monotherapy and combination matched pts were in DAS28CRP(3) remission, this increased to ≥70% from 3 months, independent of line of therapy (Fig 2).
Conclusion: Overall, RA patients persisted on UPA treatment for a median of > 2 years, with longer persistence in earlier lines of therapy. Pts initiating UPA as monotherapy or combination therapy showed similar persistence. UPA was effective irrespective of line of therapy and as either monotherapy or combination therapy, with increased remission rates as early as 3 months, reflective of observations from the UPA clinical trial program.
To cite this abstract in AMA style:
Youssef P, Ciciriello S, Tahir T, Smith T, O'Sullivan C, Leadbetter J, Butcher B, Walsh N, Calao M, Littlejohn G. Persistence, Effectiveness and Treatment Patterns of Upadacitinib in over 2600 Australian Rheumatoid Arthritis Patients: A Retrospective Analysis from the OPAL Dataset [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/persistence-effectiveness-and-treatment-patterns-of-upadacitinib-in-over-2600-australian-rheumatoid-arthritis-patients-a-retrospective-analysis-from-the-opal-dataset/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/persistence-effectiveness-and-treatment-patterns-of-upadacitinib-in-over-2600-australian-rheumatoid-arthritis-patients-a-retrospective-analysis-from-the-opal-dataset/