Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The 2010 ACR/EULAR RA criteria include autoantibodies to cyclic citrullinated peptides (CCP) that are highly specific for RA in the setting of inflammatory arthritis (IA). In addition, multiple studies demonstrate that CCP positivity can occur years prior to the onset of IA. Therefore, there is hope that CCP testing can be used to identify healthy subjects with sufficiently high risk for future RA who can be studied to understand the natural history of RA, as well as to identify subjects most likely to benefit from preventive interventions. However, while there are multiple types of CCP assays, there is limited data in subjects with and without RA comparing the performance of each assay or the ability of a positive test to predict future development of clinically-apparent RA.
Methods: CCP testing was performed using 2 commonly used ELISA assays [CCP2 (IgG) (Axis-Shield) and CCP3.1 (IgG, IgA) (INOVA)] in sera from the following: 1) RA cases from the Studies of the Etiology of RA (SERA) project, 2) RA cases from the Dept of Defense Serum Repository (DoDSR) with pre- and post-diagnosis samples available, 3) SERA first degree relatives (FDR) of probands with RA who are at elevated risk for future RA due to familial RA, and 4) controls (case-matched DoDSR subjects and randomly selected blood donors). The diagnostic accuracy of assays for established RA was calculated comparing cases (probands and DoDSR) and controls. Prevalence of positivity was compared using chi-squared/Fishers exact testing.
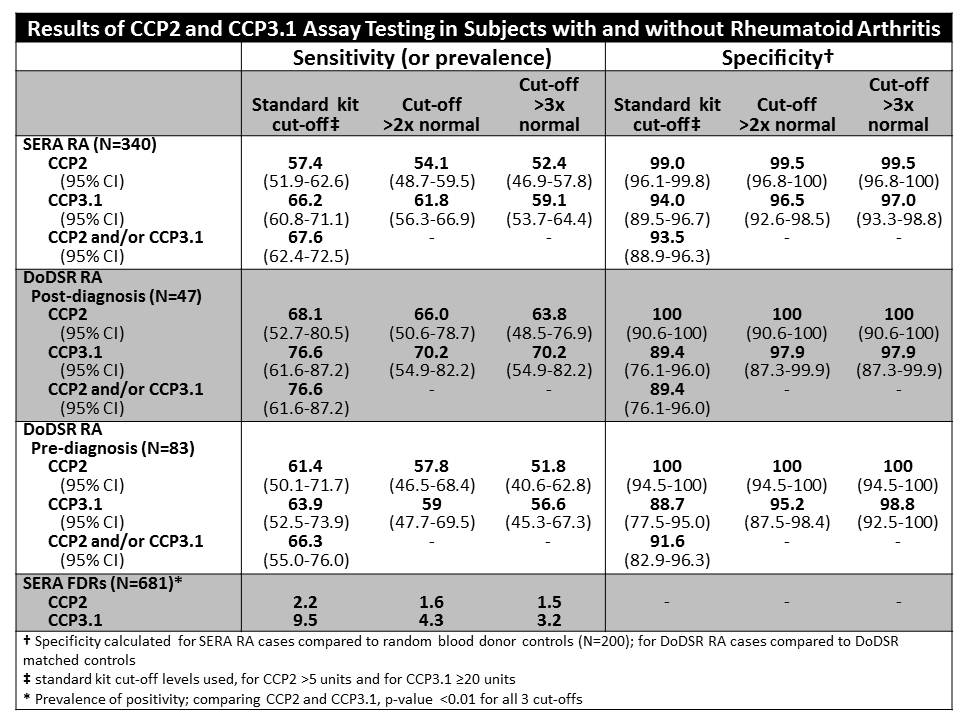
Results: For established RA, CCP2 was more specific but less sensitive than CCP3.1 (Table). As cut-off levels for positivity were increased, specificity increased. In all subjects, CCP3.1 was more prevalent than CCP2. In DoDSR samples prior to RA diagnosis, there was a strong association of CCP levels >2x normal with a diagnosis of RA within 2 years [CCP2 OR 7, 95% CI 3-17, p<0.01; CCP3.1 OR 7, 95% CI 3-16, p<0.01]. Assay agreement was good in established RA (kappa ~ 0.7), but poor in subjects without RA (k ~0.2). However, agreement improved (k ~0.5) in FDRs using cut-offs >2x normal suggesting the moderate range CCP titers encompass most of the discordant samples.
Conclusion: CCP assays differ in established RA to an extent that may have a clinically meaningful impact on diagnosis. Also, as CCP3.1 is more often elevated in pre-RA DoDSR cases and in subjects at-risk for future RA, it may represent a more sensitive test, albeit less specific, to identify subjects for investigations into the pathogenesis of RA and potential candidates for preventive interventions. Furthermore, in DoDSR cases, the association of higher CCP levels with a shorter time to the onset of RA suggests that CCP levels can be used to predict the timing of future onset of RA in currently asymptomatic subjects. These findings, as well as potential mechanisms underlying the differences (such as isotype or antigen detections) in these 2 commonly-used tests need further investigation.
Disclosure:
M. K. Demoruelle,
None;
M. Parish,
None;
L. A. Derber,
None;
M. H. Weisman,
None;
W. R. Gilliland,
None;
J. Edison,
None;
J. R. O’Dell,
None;
T. R. Mikuls,
None;
R. M. Keating,
None;
P. K. Gregersen,
None;
J. H. Buckner,
None;
J. M. Norris,
None;
V. M. Holers,
None;
K. D. Deane,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-anti-cyclic-citrullinated-peptide-assays-differs-in-healthy-subjects-at-elevated-risk-for-future-rheumatoid-arthritis-and-subjects-with-established-disease/