Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Oral ulcers, the hallmark lesion of Behçet’s syndrome (BS) can be disabling and impair eating, drinking and speaking. Despite recent advances in systemic medications for the treatment of oral ulcers, some patients do not achieve complete remission. Topical agents may help such patients by decreasing the pain and duration of oral ulcers. Pentoxifylline (PTX) is a methylxanthine derivative that inhibits phosphodiesterase and is thought to have immunomodulatory effects in addition to improving blood flow which is its main reason for use in peripheral vascular disorders. The aim of this study is to assess the efficacy and safety of PTX gel for oral ulcers in patients with BS. We also aimed to explore the best tools for the assessment of treatment response to topical agents in randomized controlled trials (Clinicaltrial.gov ID: NCT 03888846).
Methods: This was an open-label, randomized, parallel group study comparing PTX gel in addition to colchicine (PTX-COL) with colchicine alone (COL). Patients with BS who were treated with colchicine and not using any other systemic medications for BS, having at least one oral ulcer that appeared during the last 48 hours were included. PTX 5% gel with a dose of 1000 mg/day was applied in 4 divided doses per day for 14 days. Patients were contacted daily for 14 consecutive days. Photographs were taken every 24 – 48 hours and graphical processing software was used to calculate the area of the index ulcer. Duration of the index ulcer, time to start of index ulcer shrinkage, time to 50% reduction in oral ulcer pain on a 10 mm visual analog scale (VAS), change from baseline in the area of the index ulcer over time, total number of oral ulcers and adverse events were evaluated. A total of 60 patients are planned to be recruited. We present here results of the interim analysis of the first 21 patients.
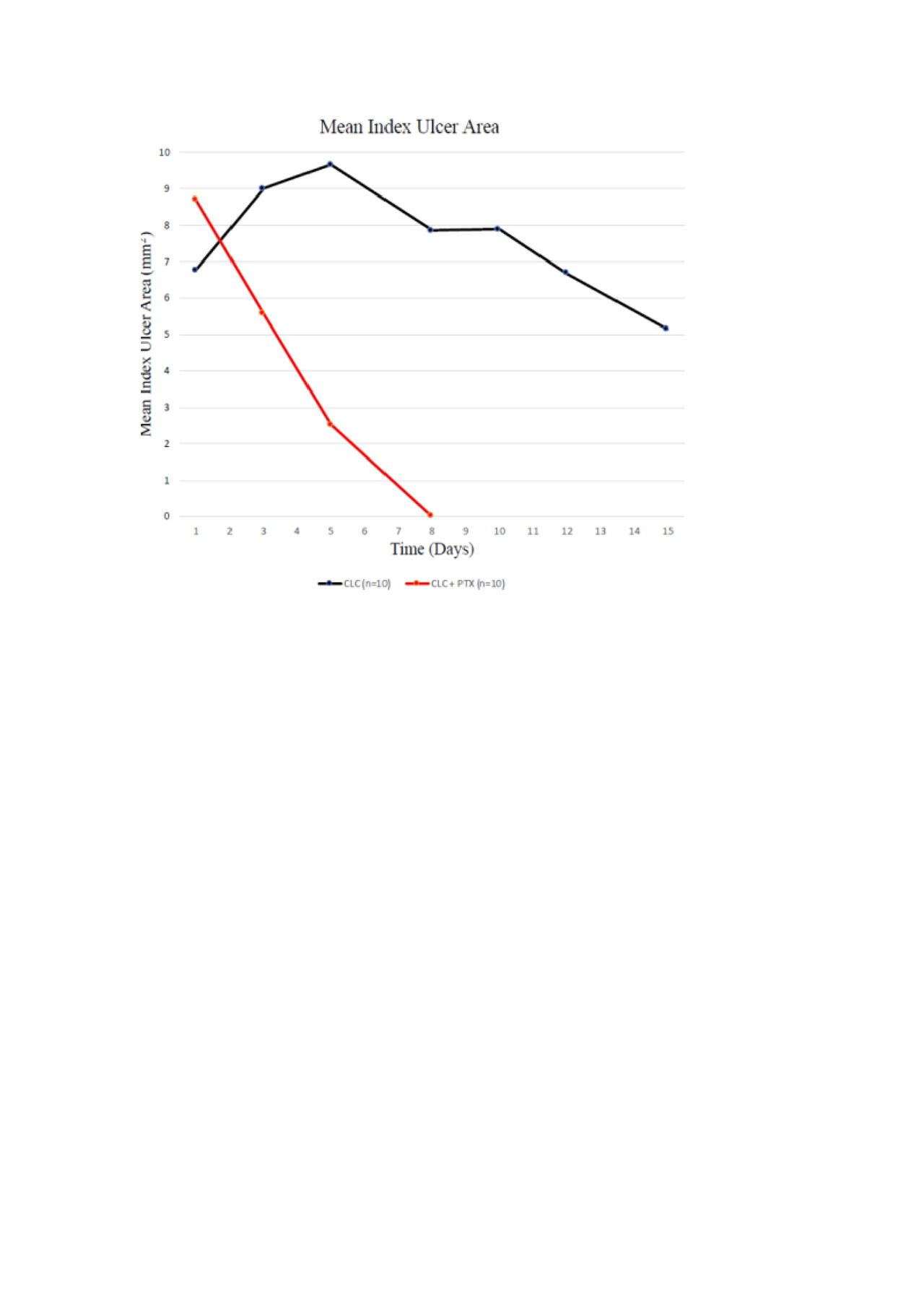
Results: A total of 21 patients (ratio M:F 1:1.1, mean age:39.9 years), 11 in the PTX-COL group and 10 in the COL group have completed the study at the time of this analysis. One patient in the PTX-COL group withdrew from the study after day 1 and was not included in the current analysis. Mean duration of index ulcer, time to start of index ulcer shrinkage, time to 50% reduction in oral ulcer pain, and total number of oral ulcers during 14 days in each group were lower in the PTX-COL group as presented in the Table. Change from baseline in the area of index ulcer over time is shown in the Figure. There were no serious adverse events. Seven patients in the PTX-COL group reported transient discomfort and nausea while they kept the gel in their mouth, 1 patient withdrew from the study for this reason.
Conclusion: The preliminary analysis of the first 21 patients of this open label, randomized trial showed that PTX gel may be a promising agent for decreasing the duration and pain of oral ulcers in patients with BS: However, caution is required when interpreting these results in a yet small number of patients. Duration of oral ulcers, time to start of ulcer shrinkage and 50% reduction in pain score seem to be relevant outcomes for studying topical agents in RCTs for oral ulcerations of BS.
To cite this abstract in AMA style:
Hatemi G, Yurttas B, Kutlubay Z, Cote T, Derkunt S, Yazici Y, Yazici H. Pentoxifylline Gel for Oral Ulcers in Patients with Behçet’s Syndrome [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pentoxifylline-gel-for-oral-ulcers-in-patients-with-behcets-syndrome/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pentoxifylline-gel-for-oral-ulcers-in-patients-with-behcets-syndrome/