Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: A variety of newer gout therapies have been introduced in recent years, some with potential safety concerns. However, uptake of these therapies and associated dose/duration of use is not clear. We used the American College of Rheumatology’s electronic health record-based registry (RISE) to examine patterns of gout drug use over time.
Methods: We used RISE data to identify gout patients by ICD9/10 diagnosis (ICD9: 274.*; ICD10: M10.*, M1A.*) with at least one physician visit in 2016, excluding patients with diagnoses for other types of inflammatory arthritis. Gout medications were identified based on written prescriptions and infusion orders and classified in the following hierarchy so as to assign uniquely: pegloticase, lesinurad, febuxostat, allopurinol, and probenecid. Baseline characteristics including demographics, comorbidities, serum uric acid, and estimated glomerular filtration rate (eGFR) were classified on the date of initiation or using the most recent preceding value. Descriptive characteristics compared initiators of various gout drugs over calendar time 2015-2018.
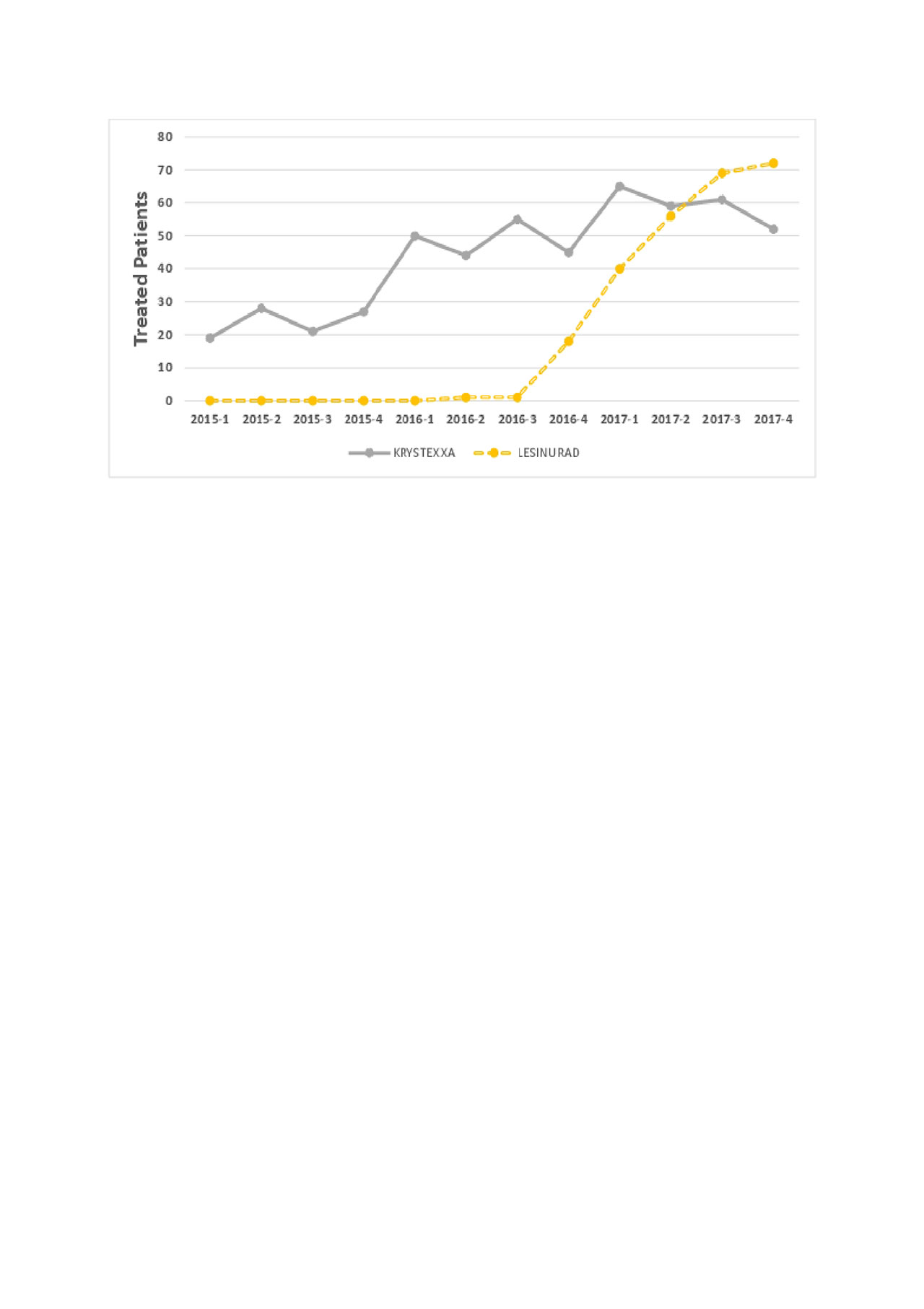
Results: Patterns of gout drug use are shown in Figures 1 and 2. Allopurinol was the most common drug used, followed by febuxostat. Krystexa and lesinurad were used much less frequently, approximately 1/200 of allopurinol use. In total, 338 patients received pegloticase, prescribed by approximately 150 unique clinicians. Of these, 53% of patients receive only 1 pegloticase dose. For those who received more than 1 dose, the median (IQR) duration of treatment was 105 (24, 294) days.
Overall a total of 19,763 met eligibility criteria for the gout cohort. Mean [SD] age was highest in the febuxostat group: 64 [14] years compared to allopurinol (63 [14]) and pegloticase (61 [16]). Black race was most common in the febuxostat group (12.3%) and lowest in allopurinol (7.8%). Febuxostat-treated patients had a higher prevalence of coronary atherosclerosis (4.8%) vs. allopurinol users (2.8%), and chronic kidney disease was more common in febuxostat patients (median eGFR 57 ml/min) vs. allopurinol patients (68 ml/min). NSAIDs were used by approximately one-third of allopurinol (36%) and febuxostat (31%) users and more than half (56%) of pegloticase patients. Colchicine was used even more frequently (allopurinol: 50%; febuxostat: 59%; pegloticase: 83%). Opioids were commonly used as concomitant treatment: 24%, allopurinol; 32%, febuxostat; and 40%, pegloticase.
Conclusion: The EHR-based ACR RISE registry appears useful to examine prescribing trends for gout medication use, even for relatively uncommonly used therapies. Febuxostat users had a higher prevalence of cardiovascular-related comorbidities compared to allopurinol users, inviting caution in the selection of patients to receive this therapy given recent FDA warnings.
Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
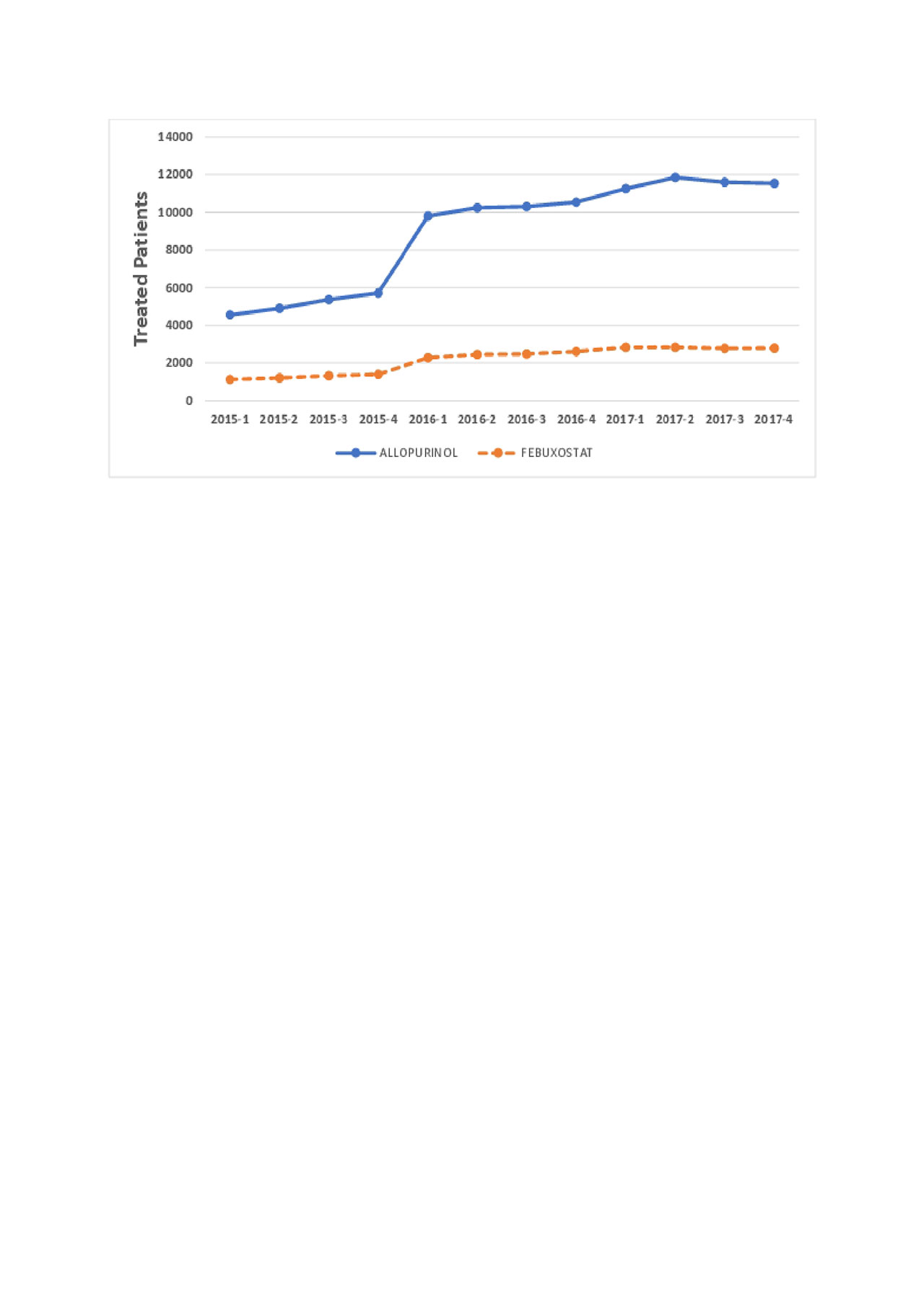
Figure 1 Gout RISE ACR 2019 v1
To cite this abstract in AMA style:
Saag K, Reimold A, Chen L, Yun H, Curtis J. Patterns of Newer Gout Medication Use in a U.S. Electronic Health Record-Based Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patterns-of-newer-gout-medication-use-in-a-u-s-electronic-health-record-based-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-newer-gout-medication-use-in-a-u-s-electronic-health-record-based-registry/