Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Etanercept (ETN) is an anti-tumor necrosis factor (anti-TNF) therapy that is FDA approved for the treatment of polyarticular juvenile idiopathic arthritis (JIA). This study describes the use of ETN in JIA patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) JIA Registry.
Methods: The CARRA Registry is a convenience cohort of children that includes patients with JIA. Retrospective data is collected at enrolment and prospective observational data is collected twice a year, as well as when a JIA medication is initiated. Data were collected from June 30, 2015 to June 30, 2018 from 60 U.S. and 3 Canadian clinical sites were included in this analysis. JIA patients treated with ETN were included if the month and year of initiation were available. Concomitant methotrexate use was described for patients who had >1 follow-up visit ≥ 6 months after beginning treatment with ETN. Descriptive statistics including means, standard deviations, medians, and interquartile ranges for continuous variables, and proportions for categorical variables were calculated as appropriate.
Results: At the time of data extraction, there were 5641 patients with JIA in the registry, and 2032 patients met inclusion criteria (74% female, median age of diagnosis 6.0 years [25th and 75th percentiles 2.0, 11.0]). Reported JIA ILAR categories were: 22.5% oligoarticular, 42.6% polyarticular RF negative (RF- pJIA), 11.9% polyarticular RF positive (RF+ pJIA), 8.1% psoriatic, 10.2% enthesitis related arthritis (ERA), 2.6% systemic, and 2.0% undifferentiated. There were 465 participants with a study visit within 30 days of starting ETN (Table 1). Within this group, ETN was started ≤3 months after diagnosis by 38% and >3 months after diagnosis by 57% (5% missing), while 62% started a non-biologic DMARD prior to ETN, primarily MTX. By ILAR category, RF+pJIA had the highest active joint count and clinical Juvenile Arthritis Disease Activity Score limited to 10 joints (cJADAS10), followed by RF-pJIA (Table 1). Fifty three percent of RF- pJIA, 44% of RF+ pJIA and 58% of ERA with a study visit within 30 days of ETN initiation started ETN >3 months after diagnosis. Table 2 shows the pattern of MTX use in individuals with at least one visit 6 months after starting ETN (n=1681). In all categories of JIA, the most common treatment approach was adding ETN after initiating MTX (step up strategy).
Conclusion: This study describes contemporary patterns of ETN use in the CARRA Registry, a North American convenience cohort that includes a large population of JIA patients. Most JIA patients started ETN >3 months after diagnosis (all categories except RF+ pJIA). A step-up strategy, with the addition of ETN to MTX, was the most common approach when starting ETN in all categories of JIA.
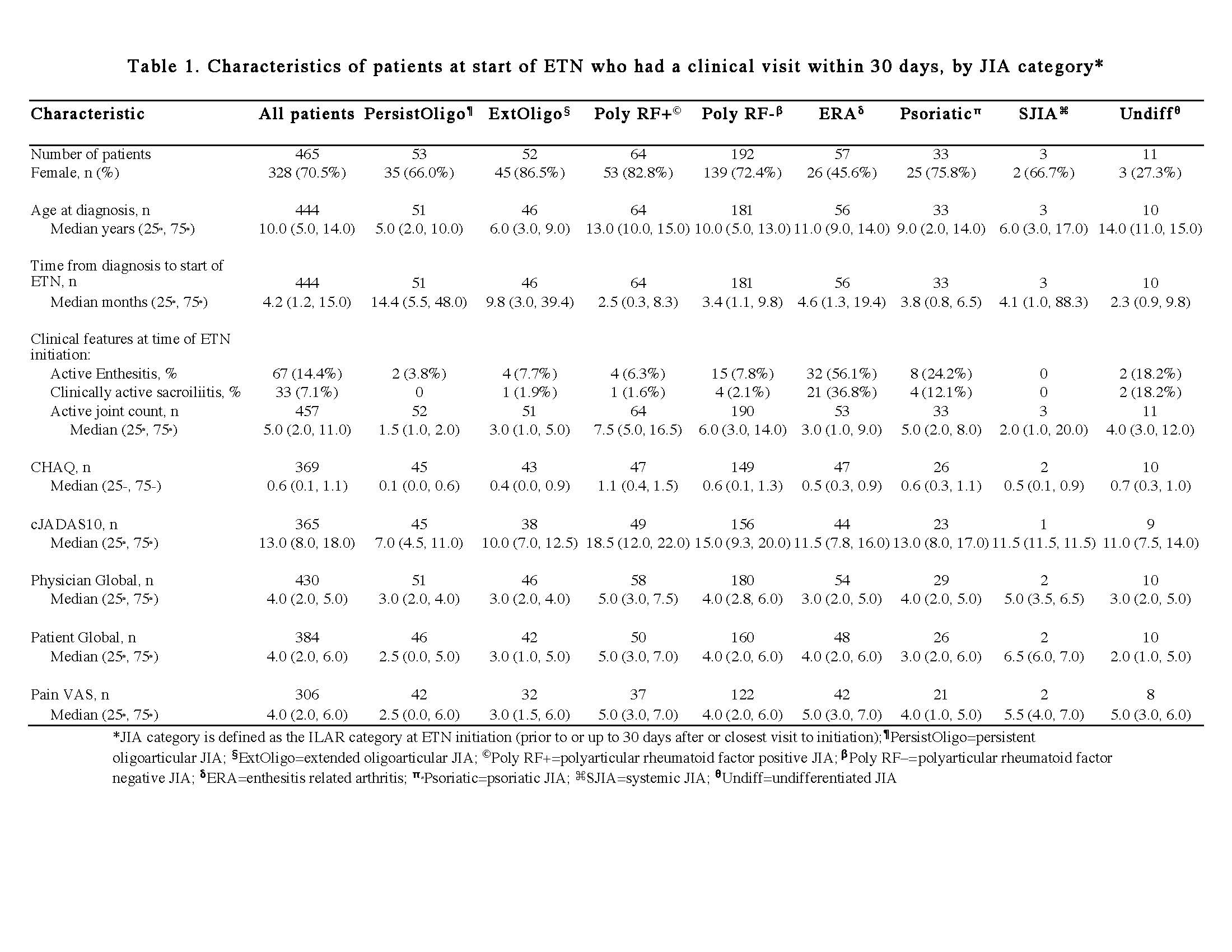
Table 1 updated formatted ACR abstract June 3 2019
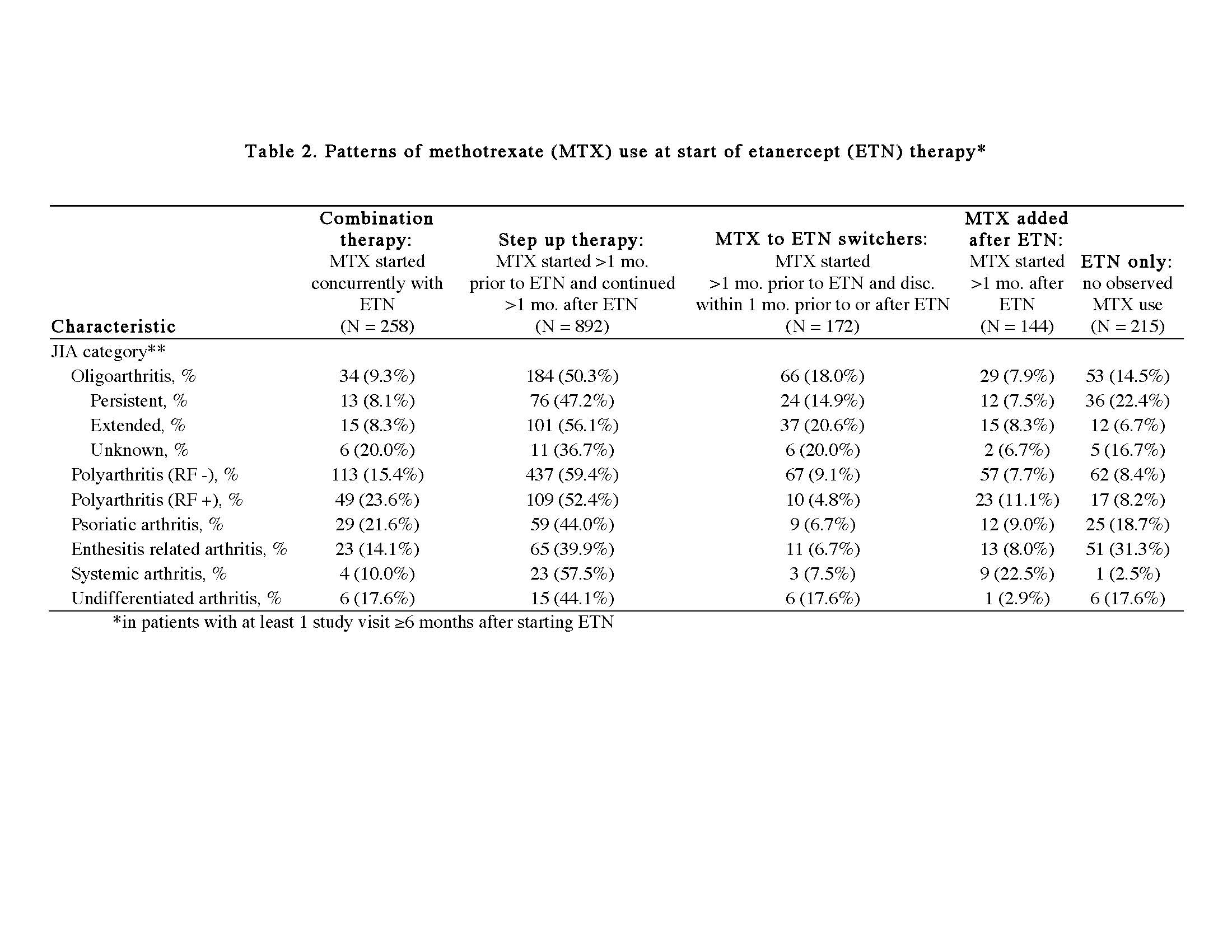
Table 2 updated ACR ETN abstract_June 3 2019
To cite this abstract in AMA style:
Shiff N, Lougee A, Matsouaka R, Collier D, Kimura Y, Rumsey D, Schenfeld J, Stryker S, Twilt M, Beukelman T. Patterns of Etanercept Use in the Childhood Arthritis and Rheumatology Research Alliance Juvenile Idiopathic Arthritis Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patterns-of-etanercept-use-in-the-childhood-arthritis-and-rheumatology-research-alliance-juvenile-idiopathic-arthritis-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-etanercept-use-in-the-childhood-arthritis-and-rheumatology-research-alliance-juvenile-idiopathic-arthritis-registry/
