Session Information
Date: Monday, October 22, 2018
Title: 4M094 ACR/ARHP Abstract: Health Services Research I: Focus on Big Data (1887–1892)
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In the U.S., the first biosimilar tumor necrosis inhibitor (TNFi) was approved in 2016. To date, only biosimilars for infliximab are available in the U.S., with limited data on biosimilar utilization. The objective of this study was to examine the early experience in prescribing patterns of biosimilar use in a U.S. based rheumatology registry.
Methods: This study was performed using the ACR’s RISE Registry consisting of electronic medical record (EMR) data from 245 rheumatology practices across the U.S. We studied data from September 9/1/2016, one month prior to the first biosimilar prescription through 3/31/18. In this time period, we studied all subjects with at least one encounter ≥6 months to the first biosimilar or TNFi prescription. Biosimilar data were identified through a combination of billing for procedures (‘J codes’) and text string searches for the two available biosimilars: infliximab-dyyb (Inflectra), infliximab-abda (Renflexis). For clarity, we refer to the trade names. Data were extracted on demographics, diagnosis codes, location of practice, and insurance. We used descriptive statistics to compare characteristics of patients who were ever treated with a biosimilar compared to Remicade.
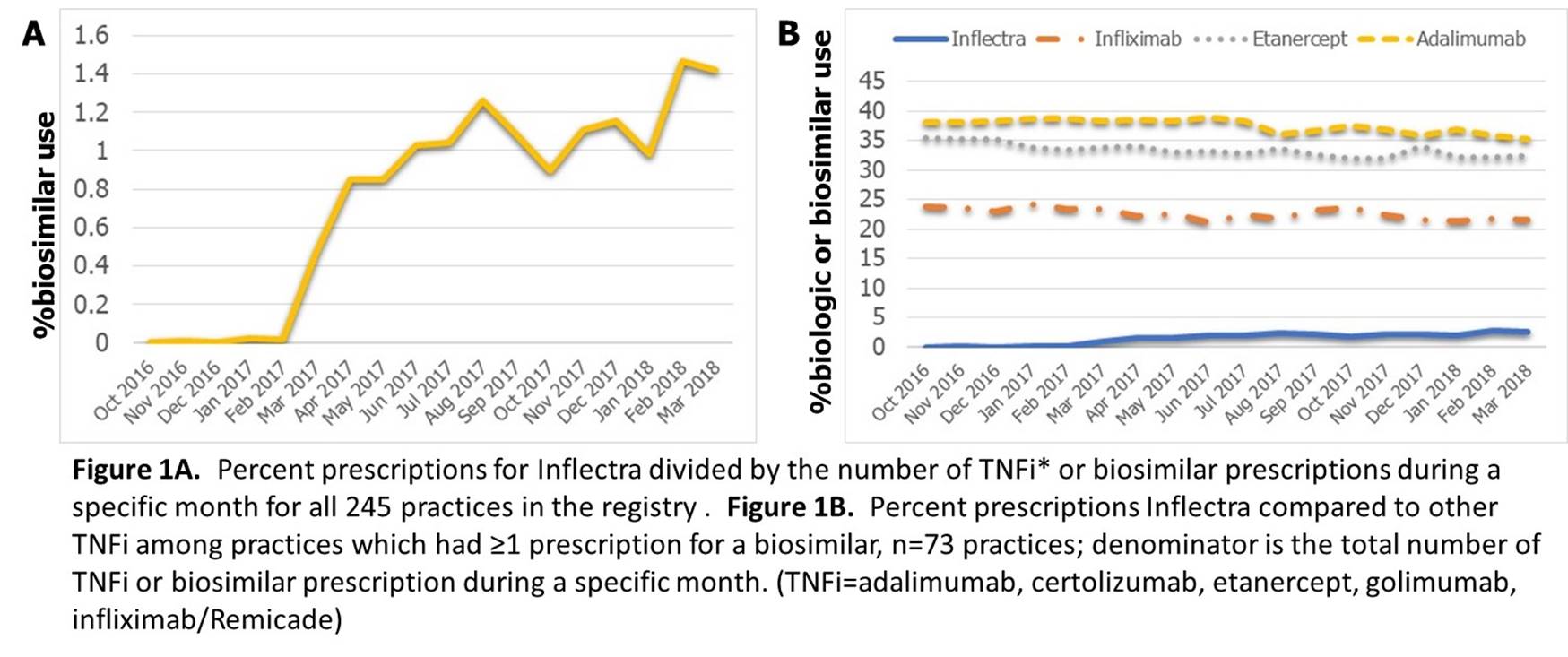
Results: At baseline, 857 patients received a biosimilar at 73 rheumatology practices (30% of all practices in RISE), all for Inflectra. Mean age of patients receiving Inflectra was 57.5 years, 70% female; characteristics were similar to those receiving Remicade (Table). A higher % of patients prescribed Inflectra had a diagnosis code for RA compared to Remicade. In absolute numbers, the highest numbers of prescriptions for Inflectra were from California (n=137), West Virginia (n=101), and Texas (n=96). Among patients who received Inflectra, 63.4% were previously on Remicade and the rest were naïve to Remicade. Over 15 months of follow-up, there were no significant differences in the types of biologics prescribed after Inflectra vs Remicade. Inflectra use increased modestly (Figure 1A), without significant change in overall TNFi usage for patients at practices who treated ≥1 patient with biosimilar-infliximab (Figure 1B).
Conclusion: This preliminary study examining the first 1.5 years after biosimilar approval in the U.S., observed a modest uptake in biosimilar usage, all for Inflectra. Nationwide rheumatology registries can provide important aggregate data on early biosimilar utilization using real world data.
Disclaimer: This data was supported by the ACR’s RISE Registry, however the views expressed represent those of the authors, not necessarily those of the ACR.
|
Table 1. Comparison of subjects initiating biosimilar-infliximab compared to infliximab October 2016 to March 2018. |
||
|
|
Inflectra |
Remicade |
|
Clinical characteristics |
n=857 |
n=4863 |
|
Age, mean years |
57.5 |
57.5 |
|
Female (%) |
70.3 |
72.7 |
|
Race (%)* |
N=781 |
N=3635 |
|
White (w) |
86.3 |
75.1 |
|
Black (b) |
4.0 |
8.8 |
|
Other (o) |
9.7 |
16.1 |
|
|
|
|
|
Diagnoses codes (%) |
|
|
|
RA |
68.5 |
48.1 |
|
Psoriasis and PsA |
20.8 |
16.7 |
|
AS |
4.6 |
8.0 |
|
|
|
|
|
Type of insurance (%)* |
n=484 |
n=2,332 |
|
Medicare |
70.7 |
41.1 |
|
Medicaid |
2.3 |
5.2 |
|
Commercial |
27.1 |
52.7 |
|
*Race and insurance was available on the subset number of individuals (n) listed. |
||
To cite this abstract in AMA style:
Bansback N, Curtis JR, Huang J, Lewis L, Johansson T, Michaud K, Liao KP. Patterns of Biosimilar Use in the Rheumatology Informatics System for Effectiveness (RISE) Registry [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/patterns-of-biosimilar-use-in-the-rheumatology-informatics-system-for-effectiveness-rise-registry/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patterns-of-biosimilar-use-in-the-rheumatology-informatics-system-for-effectiveness-rise-registry/