Session Information
Date: Friday, November 6, 2020
Title: Epidemiology & Public Health Poster I: COVID-19 & Rheumatic Disease
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Therapeutic interventions for Immune-mediated inflammatory diseases (IMIDs) target cytokines, such as TNF-a, IL-6, IL-17 and IL-23, which are involved in the physiological and pathological host response elicited by SARS-CoV-2 [1]. We therefore questioned whether IMID patients receiving cytokine inhibitors might be at lower risk for SARS-CoV-2 infection and measured the development of anti-SARS-CoV-2 IgG responses [2] in IMID patients treated with cytokine inhibitors and respective controls during the outbreak of COVID-19 in Europe.
Methods: IMID patients with stable ( > 3 months) treatment with cytokine inhibitors (TNFi, IL-6i, JAKi, IL-17i, IL-23i, IL-12/23i; N=534) were analyzed. As controls, (i) IMID patients receiving no cytokine inhibitors (N=259), healthy control subjects unrelated to health care (NHC, N=971) and health care professionals involved in the treatment of these patients (HC, N=285) were recruited. Respiratory and other infectious symptoms as well as social behavior during the outbreak of COVID-19 in Europe between February 1st to April 30th 2020 were recorded for a period of 12 weeks. To test for exposure to SARS-CoV-2 we used an S1 domain-based antibody ELISA for the initial testing (commercial enzyme–linked immunosorbent assay from Euroimmun (Lübeck, Germany)). A cut-off of ≥0.8 (OD450nm) was considered as positive. Relative risks (RR) of seropositivity in study groups using the NHC group as the reference and adjusting for age, sex and sampling-date using a Poisson regression model with robust sandwich standard errors weres estimated. Adjustment for sampling date was achieved using the cumulative confirmed COVID-19 case-counts in the region where the study was conducted.
Results: Positive anti-SARS-CoV-2 IgG was observed in 22 of 971 NHC with a prevalence (95%CI) of 2.27% (1.42% to 3.43%), 12 of 285 HC with a prevalence of 4.21% (2.18% to 7.25%), 8 of 259 IMID patients without cytokine blockade with a prevalence of 3.09% (1.33% to 6.09%) and 4 of 534 IMID patients under cytokine blockade with a prevalence of 0.75% (0.20% to 1.92%) (Fig. 1B). After age-, sex- and sampling date adjustment, the prevalence of anti-SARS-CoV-2 IgG was significantly lower (RR: 0.32, 95%CI: 0.11 to 0.99, p=0.048) in IMID patients receiving cytokine inhibitors compared to NHC. In contrast, IMID patients receiving no cytokine inhibitors had similar (RR: 1.21, 95%CI: 0.50 to 2.90, p=0.676) prevalence of anti-SARS-CoV-2 IgG. Standardized Pearson’s residuals of the expected frequencies for self-reported contact with individuals having a respiratory tract infection, workplace attendance and travel to risk areas were similar in IMID patients with and without cytokine inhibitors (Fig. 1C).
Conclusion: Patients with IMIDs receiving cytokine inhibitors are not at enhanced but rather at lower risk for SARS-CoV-2 infection compared to the general community and IMID patients not receiving such drugs. These data support a pathogenic role of cytokines, targeted by treatment of IMIDs, in COVID-19 and clearly speaks against stopping cytokine inhibitor treatment in patients with IMIDs during the current SARS-CoV-2 pandemic.
[1] Schett et al. Nat Rev Immunol. 2020
[2] Okba et al. medRxiv; doi:
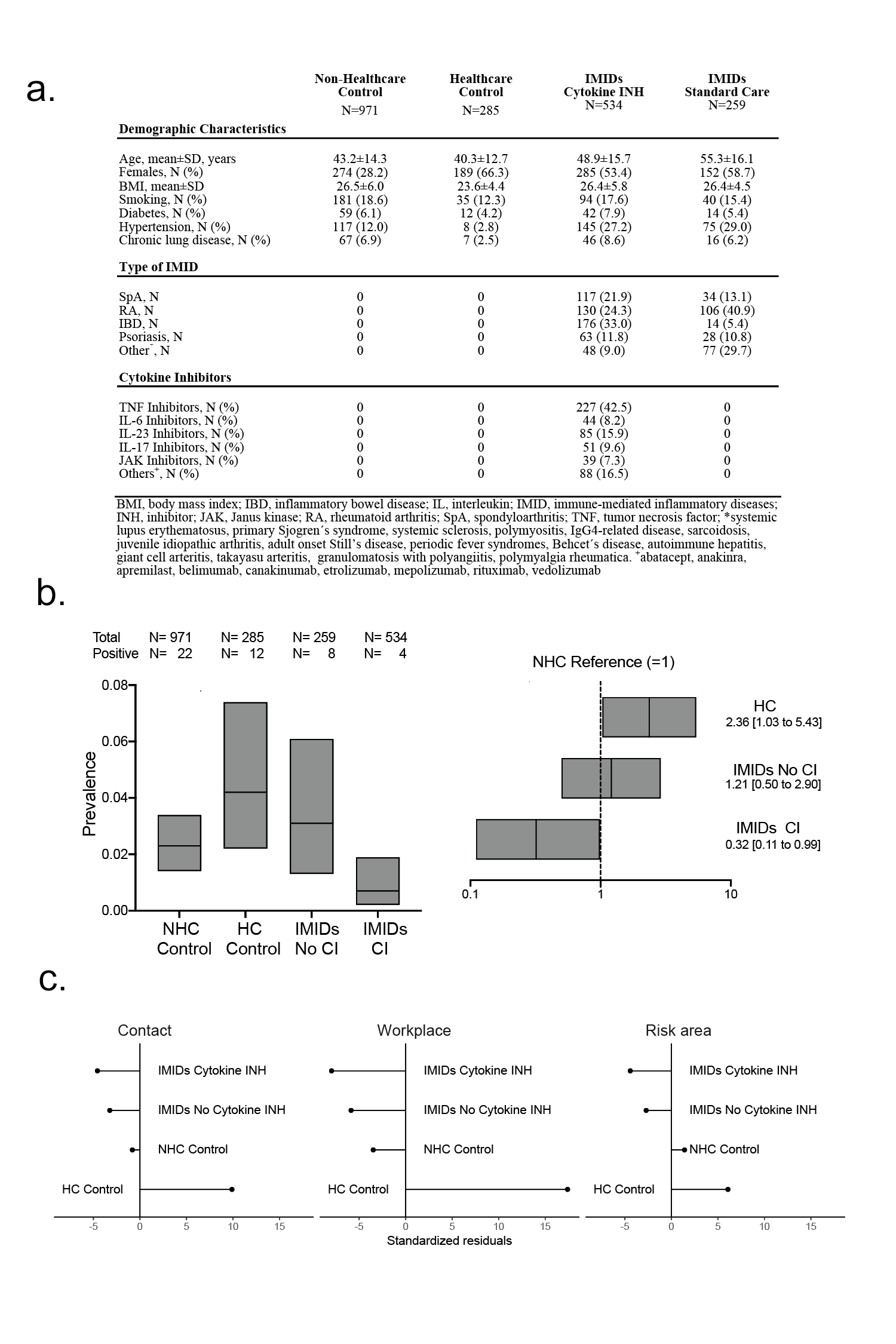 (a) Characteristics of the patients with IMIDs and controls. (b) left side: Prevalence and 95% confidence intervals of a positive anti-SARS-CoV-2 IgG antibody test recognizing the S1 domain of the spike protein in non-health care (NHC) controls, health care (HC) controls and IMIDs with and without cytokine inhibitors (CI); right side: risk ratios and 95% confidence intervals of anti-SARS-CoV-2 IgG antibody positivity in health care (HC) controls and IMIDs with and without cytokine inhibitors (CI) with NHC controls as reference; (c) Standardized Pearson’s residuals showing deviation from the expected frequencies for exposure risk variables (contact with persons with respiratory infections, presence at workplace outside home, travel to risk areas) of IMID patient groups and control groups.
(a) Characteristics of the patients with IMIDs and controls. (b) left side: Prevalence and 95% confidence intervals of a positive anti-SARS-CoV-2 IgG antibody test recognizing the S1 domain of the spike protein in non-health care (NHC) controls, health care (HC) controls and IMIDs with and without cytokine inhibitors (CI); right side: risk ratios and 95% confidence intervals of anti-SARS-CoV-2 IgG antibody positivity in health care (HC) controls and IMIDs with and without cytokine inhibitors (CI) with NHC controls as reference; (c) Standardized Pearson’s residuals showing deviation from the expected frequencies for exposure risk variables (contact with persons with respiratory infections, presence at workplace outside home, travel to risk areas) of IMID patient groups and control groups.
To cite this abstract in AMA style:
Simon D, Tascilar K, Krönke G, Kleyer A, Zaiss M, Heppt F, Meder C, Atreya R, Klenske E, Dietrich P, Abdullah A, Kliem T, Corte G, Morf H, Leppkes M, Kremer A, Ramming A, Pachowsky M, Schuch F, Ronneberger M, Kleinert S, Maier C, Hueber A, Manger K, Manger B, Berking C, Tenbusch M, Überla K, Sticherling M, Neurath M, Schett G. Patients Receiving Cytokine Inhibitors Have Low Prevalence of SARS-CoV-2 Infection [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/patients-receiving-cytokine-inhibitors-have-low-prevalence-of-sars-cov-2-infection/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-receiving-cytokine-inhibitors-have-low-prevalence-of-sars-cov-2-infection/
