Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Lupus nephritis can occur as an isolated component of disease activity or be accompanied by diverse extrarenal symptoms that can adversely affect a patient’s quality of life (QOL). Whether renal disease absent other activity is sufficient to decrease QOL is unknown. A lack of reported QOL impairment may place patients at risk for delayed diagnosis of nephritis or medication noncompliance yet nephritis trials have largely neglected QOL. As such, this study leveraged the multi-center multi-racial Accelerating Medicines Partnership (AMP) lupus nephritis cohort to assess QOL measured by PROMIS-29.
Methods: Patients (n=182) fulfilling ACR or SLICC criteria for SLE with a uPCR > .5 and biopsy Class III, IV, V, or mixed were consecutively enrolled in AMP at the time of renal biopsy and clinical history, PROMIS-29, and disease activity as assessed by the hybrid SELENA-SLEDAI were recorded. Patients were determined to have extrarenal clinical activity if, after excluding all laboratory parameters from the SLEDAI, the score remained > 1. Raw PROMIS-29 scores were transformed to t-scores with the mean of 50 + 10 representing the US population and a difference of 5 points considered clinically meaningful. PROMIS-29 physical and mental health summary scores were calculated according to published formulas.
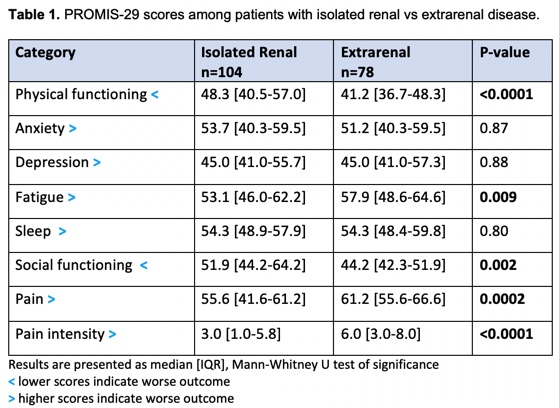
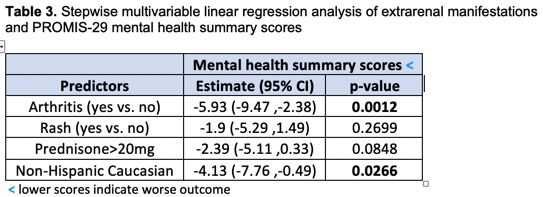
Results: Forty-three percent of patients (n=78) had extrarenal clinical manifestations including vasculitis (4%), arthritis (39%), rash (45%), alopecia (42%), mucosal ulcers (13%), pleurisy (12%), pericarditis (8%), and fever (4%). Patients with isolated renal disease (n=104, 57%) did not have PROMIS-29 scores that differed clinically from the US population whereas patients with extrarenal disease reported deficits in physical functioning, fatigue, social functioning, and pain (Table 1). Patients with extrarenal disease had significantly lower physical health summary scores compared to patients with isolated disease (median [IQR]: 40.31 [35.79, 47.02] p< 0.001 vs. 48.6 [40.14, 57.08]) and significantly lower mental health summary scores (44.12 [38.63, 51.39], p=0.024 vs. 48.67 [40.51, 55.07]). Female and African American patients and those with nephrotic range proteinuria or undergoing first biopsy had significantly lower physical health summary scores, but mental health summary scores did not differ by these variables. Patients on greater than 20 mg of prednisone had both significantly lower physical and mental health summary scores compared to those on lower doses. PROMIS-29 scores did not differ by low complements, anti-dsDNA, or anti-Ro antibodies. Stepwise multivariable linear regression analysis demonstrated that the association between extrarenal disease and lower PROMIS-29 summary scores was primarily driven by arthritis and independent of potential confounders (Tables 2 and 3).
Conclusion: The majority of patients had isolated renal disease and report a QOL similar to that of the general population. In contrast, those with extrarenal manifestations report significantly worse QOL outcomes. These results reinforce the critical importance of routine laboratory surveillance and medication compliance for nephritis even in patients with seemingly quiescent clinical disease.
To cite this abstract in AMA style:
Carlucci P, Li J, Gold H, Deonaraine K, Fava A, Buyon J, James J, Putterman C, Rao D, Diamond B, Fine D, Monroy-Trujillo J, Haag K, (AMP) RA/SLE Network A, Belmont H, Connery S, Payan-Schober F, Furie R, Berthier C, Dall'Era M, Cho K, Kamen D, Kalunian K, In SLE Network T, Izmirly P, Petri M. Patients Enrolled in the Accelerating Medicines Partnership (AMP) RA/SLE Network with Isolated Renal Disease Report Minimal Quality of Life Impairment on PROMIS-29 Compared to Patients with Extrarenal Symptoms [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/patients-enrolled-in-the-accelerating-medicines-partnership-amp-ra-sle-network-with-isolated-renal-disease-report-minimal-quality-of-life-impairment-on-promis-29-compared-to-patients-with-extrarenal/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-enrolled-in-the-accelerating-medicines-partnership-amp-ra-sle-network-with-isolated-renal-disease-report-minimal-quality-of-life-impairment-on-promis-29-compared-to-patients-with-extrarenal/