Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The Brazilian public healthcare system covers treatment with adalimumab for rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis and psoriatic arthritis, in line with local guidelines. Patients treated with adalimumab in Brazil can opt-in to a Patient Support Program (PSP) offered by AbbVie, which includes elements such as nurse and telephone support. The impact of this program on patient outcomes in the Brazilian setting has not been studied previously. The objective of the study is to evaluate the relationship of PSP enrollment and treatment utilization outcomes (adherence and persistence) among patients who initiate adalimumab.
Methods: Longitudinal data on the utilization of AbbVieÕs PSP were linked with the Brazilian Health System claims database called DATASUS, which includes data on patients that initiated treatment with adalimumab between 2013 and 2015. Patients using adalimumab in DATASUS not matched with AbbVie PSP database were categorized as non-users (non-PSP). Adherence was calculated using proportion of days covered (PDC), defined as the number of months of treatment with adalimumab divided by the number of months of patient follow-up. Patients were considered adherent if they had a PDC ³ 80%1. Persistence was calculated as the interval between treatment initiation and treatment discontinuation (defined as a gap of 90 days since the last obtainment of adalimumab). Adherence and persistence were compared between the PSP and non-PSP cohorts using t tests.
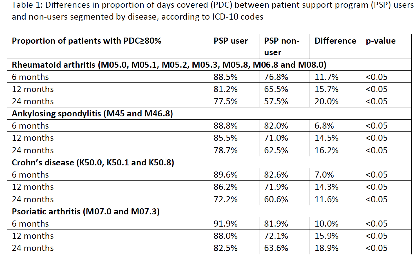
Results were segmented for patients with at least 6, 12 and 24 months follow-up. Results: 21,690 patients were included in the analysis: 3,313 in the PSP cohort and 18,377 in the non-PSP cohort. Patient characteristics were similar between groups: 63% were female, with an average age of 47 years (± 14.6). The percentages of patients with a PDC ³ 80% in the PSP vs non-PSP cohorts were: 89.4% vs 79.6% (p<0.05) in 6 months, 84.3% vs 68.6% (p<0.05) in 12 months and 77.6% vs 59.8% (p<0.05) in 24 months. The average treatment persistence in the PSP vs non-PSP groups were: 5.87 vs 5.73 months (p<0.05), 11.65 vs 11.05 months (p<0.05) and 22.37 vs 20.30 months (p<0.05) in the 6, 12 and 24 month time periods, respectively. Subgroup analyses by clinical indication showed consistent findings, in favor of the PSP, as displayed in Table 1.
Conclusion: Across the indications studied, adalimumab users participating in the AbbVieÕs free-to-patient PSP demonstrated improved adherence and persistence to treatment compared to patients not participating in the PSP.
To cite this abstract in AMA style:
Levy RA, Teich V, Fernandes R, Gulart A, Chaves L, Garg V, Skup M. Patient Support Program for Adalimumab-Treated Patients in Brazil: Impact on Patients’ Adherence and Persistence [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/patient-support-program-for-adalimumab-treated-patients-in-brazil-impact-on-patients-adherence-and-persistence/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-support-program-for-adalimumab-treated-patients-in-brazil-impact-on-patients-adherence-and-persistence/