Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported
outcomes (PRO) are used to assess patient related benefit in clinical trials. Rheumatoid
arthritis (RA) patients are equally receptive to intravenous (IV) or
subcutaneous (SC) biologic treatment1. The objective of this study was
to obtain PRO data to understand characteristics of patients who receive IV
biologic agents for RA.
Methods: This was a questionnaire-based
study conducted at a single rheumatology practice with extensive clinical trial
experience. A total of 100 patients will be enrolled; 87 patients are included
in this interim analysis. The inclusion criteria were: a diagnosis of RA with
IV biologic use for ≥3 mo; ≥18 yrs age; able to read, write and
speak English, willing to complete the questionnaire and a signed informed
consent form. IV biologic treatment was per clinical practice; there were no
treatment assignments and study drug was not supplied. The questionnaire had 30
questions; patients completed the questionnaire prior to receiving a regularly
scheduled dose of IV biologic. Data collected 1-2Q2015.
Results: The mean (±SD) age of patients in
this interim analysis was 57.9 (±14.5) yrs with a mean disease duration of 10.6
(±8.5) yrs (range 0.7-45 yrs). Patients were Caucasian (36.8%), African
American (28.7%), Latino/Hispanic (20.7%), Asian/Pacific Islander (1.1%) and 12.6%
not identified. IV biologics used were infliximab (68%), rituximab (13%), tocilizumab
(10%), abatacept (7%), golimumab (1%) and other (1%). The mean duration of current
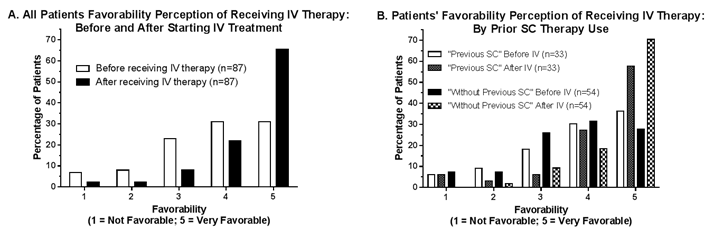
IV therapy was 4.0 (±3.2) yrs (range 0.1 to 16.0 yrs). Patients’ favorability (1=Not
Favorable, 5=Very favorable) perception of IV therapy BEFORE and AFTER starting
IV therapy is shown in Figures A and B. Amongst all
patients (A), “Very Favorable” increased (p<0.05) from 31% to 65% after
receiving IV therapy; the increase in “Very Favorable” was evident in patients with
or without previous SC therapy (B).
The favorability scores (mean ±SD) of current IV
therapies were: infliximab 4.7 (±0.6), abatacept 4.7 (±0.5), rituximab 4.3 (±1.0),
golimumab 5 (n=1) and tocilizumab 3.9 (±1.5). Prior SC biologic users had similar
responses to questions identifying advantages or disadvantages associated with
receiving IV biologic therapy as those patients who were not previous SC
biologic users.
Conclusion: These interim results suggest that among patients
receiving IV biologic therapy for treatment of RA, there is a high degree of
patient satisfaction, including a similar favorability perception of IV therapy
among patients who switched from an SC to an IV biologic. Our results support
the concept that when there is a shared decision making discussion with
patients regarding biologic treatments, the option of IV therapies should be an
essential part of that discussion and that the RA patient’s perspective should be given meaningful consideration.
1Bolge SC et al Arthritis Rheum 2013;65
Suppl 10:1023
To cite this abstract in AMA style:
Gaylis NB, Sagliani J, Tuccitto D, Black S, Tang K, Dehoratius R, Parenti D. Patient Reported Outcome Assessment of Rheumatoid Arthritis Patients Experience with IV Administered Biologic Therapy [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/patient-reported-outcome-assessment-of-rheumatoid-arthritis-patients-experience-with-iv-administered-biologic-therapy/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcome-assessment-of-rheumatoid-arthritis-patients-experience-with-iv-administered-biologic-therapy/