Session Information
Date: Monday, November 8, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster III: Patient Preferences (1153–1169)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Although hydroxychloroquine (HCQ) has been shown to reduce SLE flares, concerns exist regarding side effects from long-term use. Very little information is available on patient preferences regarding decisions to continue, lower, or stop the drug. To address these knowledge gaps, we evaluated patient experiences and preferences for HCQ therapy and qualitatively assessed themes underlying these preferences.
Methods: Telephone interviews were conducted with patients recruited from two Canadian SLE clinical cohorts. The interviews were conducted in English and French using a standardized script. The interview recordings were transcribed, and French transcripts were translated to English. Two reviewers conducted a thematic analysis by individually generating codebooks and mutually ruling out discrepancies.
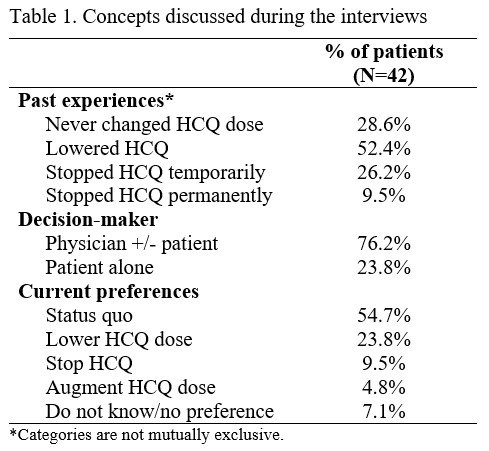
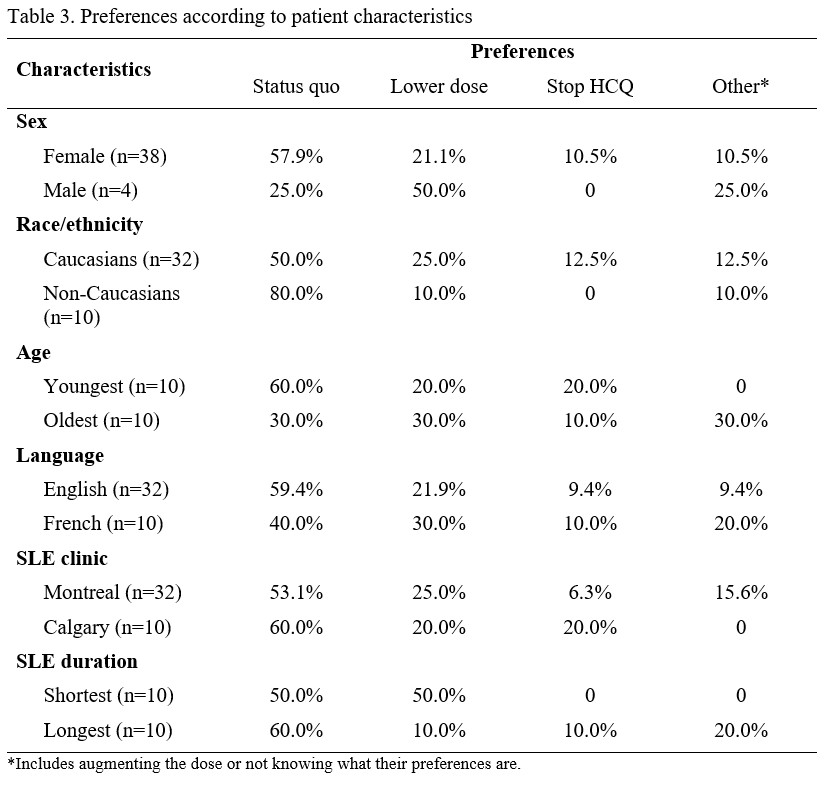
Results: We completed a thematic analysis of 42 interviews. The majority (N=38, 90%) of subjects were female. Average age was 55.7 (SD 13.0) and most subjects (N=32, 76%) were Caucasian; the rest were Black (N=4, 10%), Asian (N=4, 10%) and other races/ethnicities (N=2, 5%). Concepts emerging from interviews centred around past experiences with dose changes, who (patient/physician) makes those decisions, and current patient preferences (Table 1). Over the course of SLE, 35.7% of patients had stopped and 52.4% had lowered HCQ. Most decisions were made by physicians, but almost a fourth of patients had made a decision to lower or stop HCQ without communicating with their doctor. Though most patients preferred their dose at status quo (to avoid flares), over a third were interested in lowering, stopping, or increasing HCQ. Themes around preferences included SLE control, trust in the provider’s decisions, management of other medications, HCQ safety, and HCQ effectiveness (Table 2). We observed some differences in preferences across patient characteristics (Table 3). A higher proportion of non-Caucasians (80% vs 50% of Caucasians) and of the 10 youngest patients (60% vs 30% of the 10 oldest patients) preferred maintaining their current dose. Concerns about toxicities emerged in the interviews (Table 2). A limitation of our study is that most of our patients had long-established disease, with only about 10% having more recent-onset disease.
Conclusion: In this sample, most patients preferred maintaining their current HCQ dose to avoid SLE flares. However, concerns about toxicities emerged in the interviews, and several patients would prefer decreasing or stopping treatment, but respected physician recommendations. Awareness of these results may help patients and physicians navigate shared decision making. Studies like ours could also contribute to making SLE treatment guidelines more patient-centred and personalized.
To cite this abstract in AMA style:
Dollinger J, Brasil C, Wong M, Hazlewood G, Dollinger R, Singer W, Pineau C, Vinet E, Clarke A, Lee J, Bernatsky S. Patient Preferences for Hydroxychloroquine in Systemic Lupus (SLE) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/patient-preferences-for-hydroxychloroquine-in-systemic-lupus-sle/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-preferences-for-hydroxychloroquine-in-systemic-lupus-sle/