Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: In the COVID era, telemedicine has assumed an increasing role in rheumatology care, yet questions remain about its comparative effectiveness and satisfaction relative to in-person care, which we are studying in a randomized clinical trial (RCT) entitled EVOLVE (NCT04704544). Conducting clinical trials on telemedicine are limited by possible participation bias. We sought to describe and compare the characteristics of patients who enrolled in EVOLVE vs. those who were contacted but did not enroll at one academic medical center in the Deep South.
Methods: We used the rheumatology clinic appointment list to identify established patients age ≥18 years with a rheumatic disease with at least 2 clinic visits including ≥1 in-person visit in the last 18 months. Research assistants reviewed electronic health records of potentially eligible patients and contacted them by phone to assess acceptability of participating in either a telemedicine or in-person visit with their rheumatologist. Patients without equipoise for either visit type were excluded. We used descriptive statistics and reported mean (SD), median (interquartile range) or frequency (percentage) to summarize patient characteristics. In addition to age, sex, and race/ethnicity, we analyzed the association of covariates with enrollment status including socioeconomic disadvantage (Area Deprivation Index), distance from a participant’s primary residence to clinic, and medication use. Variables with p≤0.3 in the univariable analysis or of biologic significance (e.g., sex and race/ethnicity) were considered in a multivariable logistic regression model to examine factors associated with RCT enrollment. This project was approved by the UAB Institutional Review Board with a waiver of informed consent (IRB-300006553).
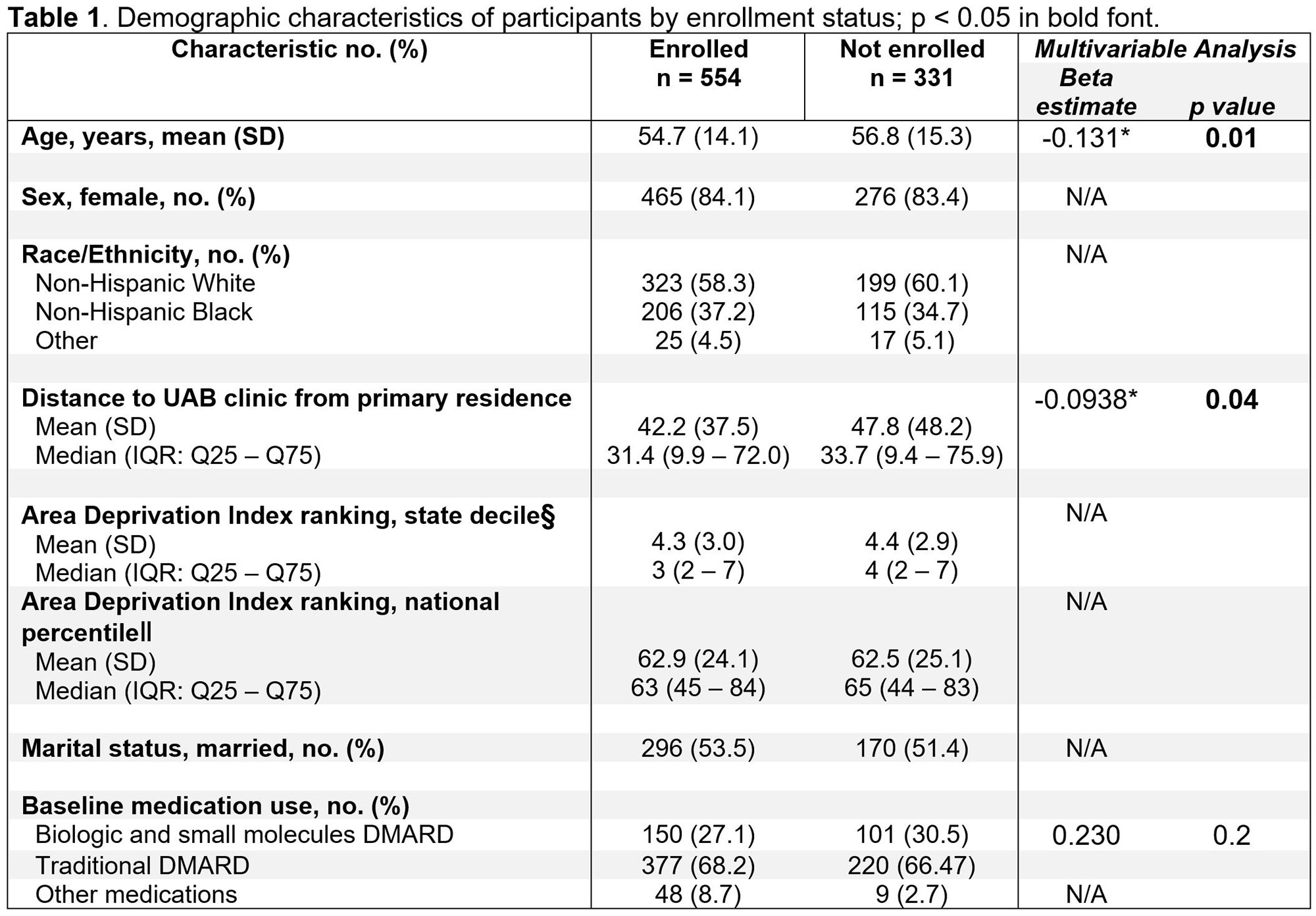
Results: From August 2021 to May 2022, 885 patients were contacted by phone. Of these, 331 persons, mean (SD) age 56.8 (15.3) years, 17% men, 34.7% non-Hispanic Blacks were not enrolled in the study (Table 1). Reasons for exclusion included patient preference for a visit type, travel distance to clinic, not wanting to be surveyed post-clinic visit, reported need for an in-office procedure, among others. Those who were enrolled vs. not were well balanced by sex, race/ethnicity, and level of socioeconomic disadvantage. In multiple regression analysis in which the outcome was study enrollment, every 10 years of increased age was associated with a 12.3% decrease in chance of enrolling (p=0.01) and every 25 miles of increased distance from clinic was associated with a 8.9% decrease in chance of enrolling (p=0.04) in the RCT.
Conclusion: We found that patients who enrolled in the EVOLVE RCT of telemedicine vs. in-person visits were younger and lived closer to the rheumatology clinic. Our findings suggest that older individuals and those living further from our academic medical center may be underrepresented in this telemedicine trial, however enrollment is ongoing. This study also illustrates good generalizability to other groups, such as those characterized by sex, race/ ethnicity, and area deprivation index. These data will inform recruitment efforts for clinical trials involving telemedicine.
§Area Deprivation Index State decile from 1 (least disadvantaged) to 10 (most disadvantaged); ‖National percentile from 1 (least disadvantaged) to 100 (most disadvantaged), missing for 120 participants. Race/ethnicity “Other” includes Hispanic/Latino, Asian, Native American, American Indian or Alaska Native, or not listed. Other medications include allopurinol, colchicine, IVIG, and anti-fibrotics (nintedanib, pirfenidone). Other medications were not included in the multivariable model due to small sample size.
To cite this abstract in AMA style:
Jackson L, Aaron K, Yazdany J, Goglin S, Perez D, Chae D, Margaretten M, Mugeta F, Techarukpong N, McNeeley E, Cutter G, saag k, Danila M. Patient Factors Influencing Recruitment in a Telemedicine Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/patient-factors-influencing-recruitment-in-a-telemedicine-randomized-clinical-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-factors-influencing-recruitment-in-a-telemedicine-randomized-clinical-trial/