Session Information
Date: Sunday, November 12, 2023
Title: (0325–0344) Patient Outcomes, Preferences, & Attitudes Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: We evaluated participants’ adherence to protocol-assigned capture of patient-reported outcomes (PROs) and activity tracker data in the context of an ongoing longitudinal study collecting data from rheumatoid arthritis (RA) patients initiating a new biologic or JAKi.
Methods: RA patients in moderate or high disease activity (CDAI >10) and prescribed either upadacitinib (UPA) or adalimumab (ADA) were enrolled during in-office visits at US community rheumatology clinics. To enter the 12-week main study, participants had to successfully complete a 7-day run-in (completion of ≥ 5 of 7 days of daily and weekly PROs) and take their first dose of new treatment within 30 days. Participants completed PROs remotely via app during the main study according to a pre-specified rotating daily assessment schedule that repeated each week and that was anticipated to take 1-3 minutes per day. As an optional component of the study, participants were provided with a Fitbit Versa2 (wearable) device either in clinic or received it by mail.
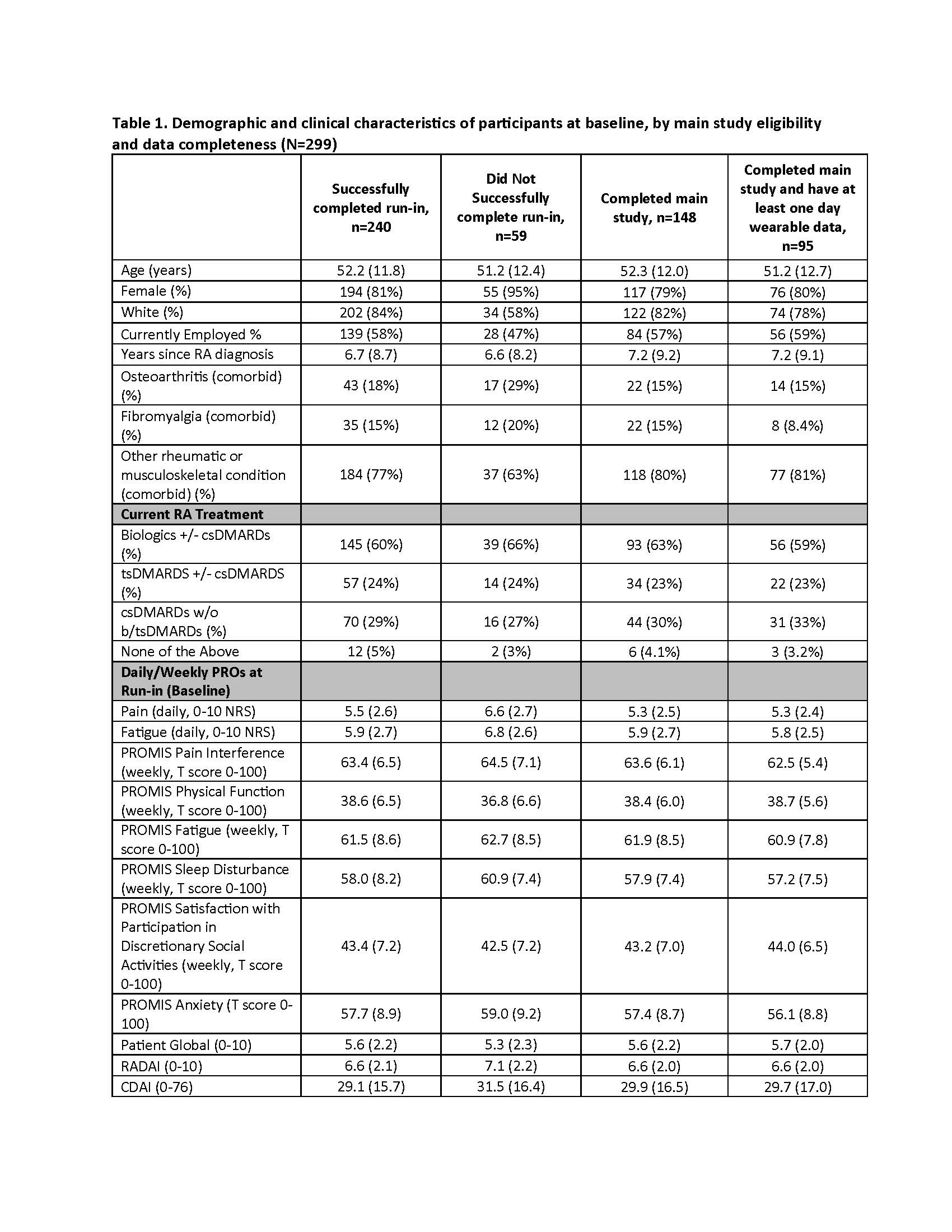
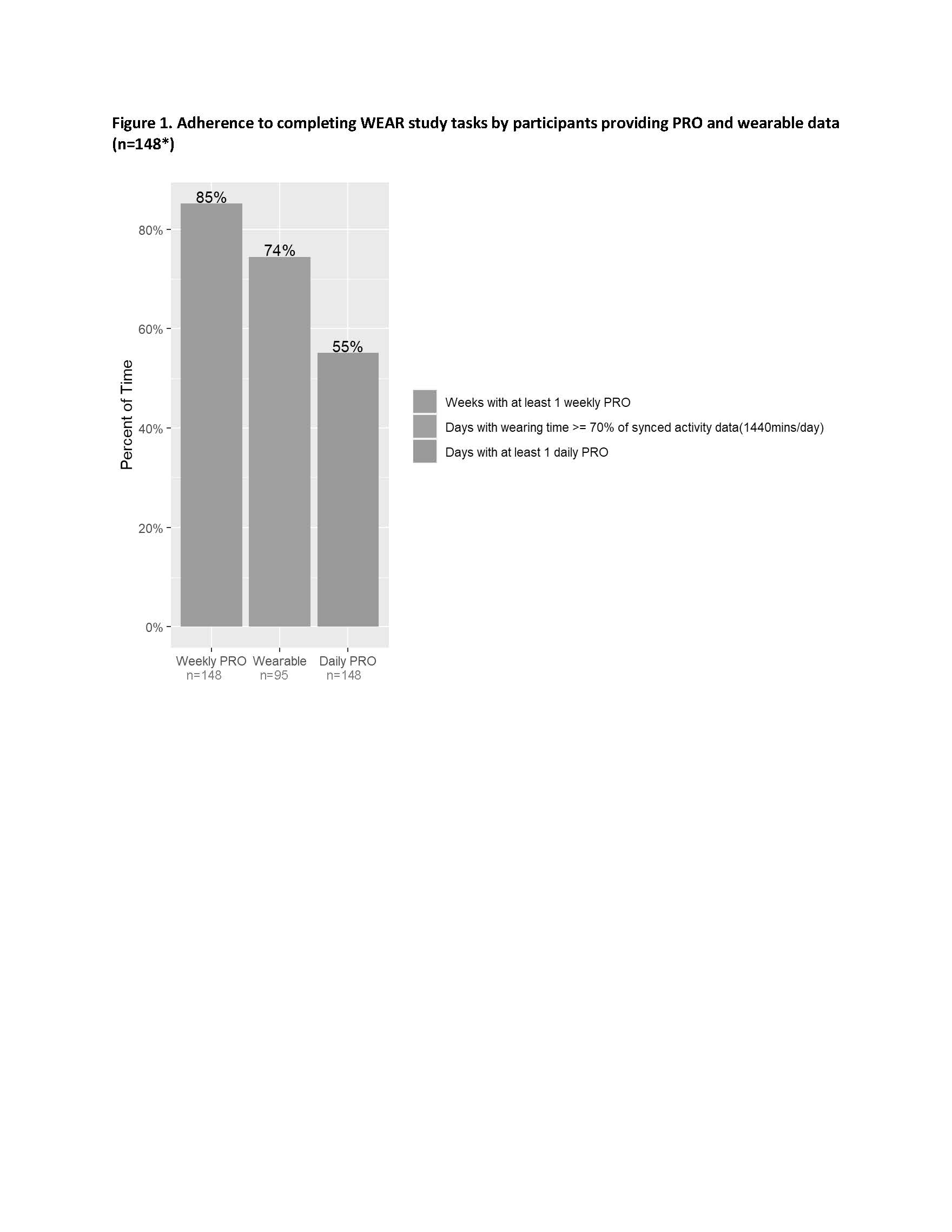
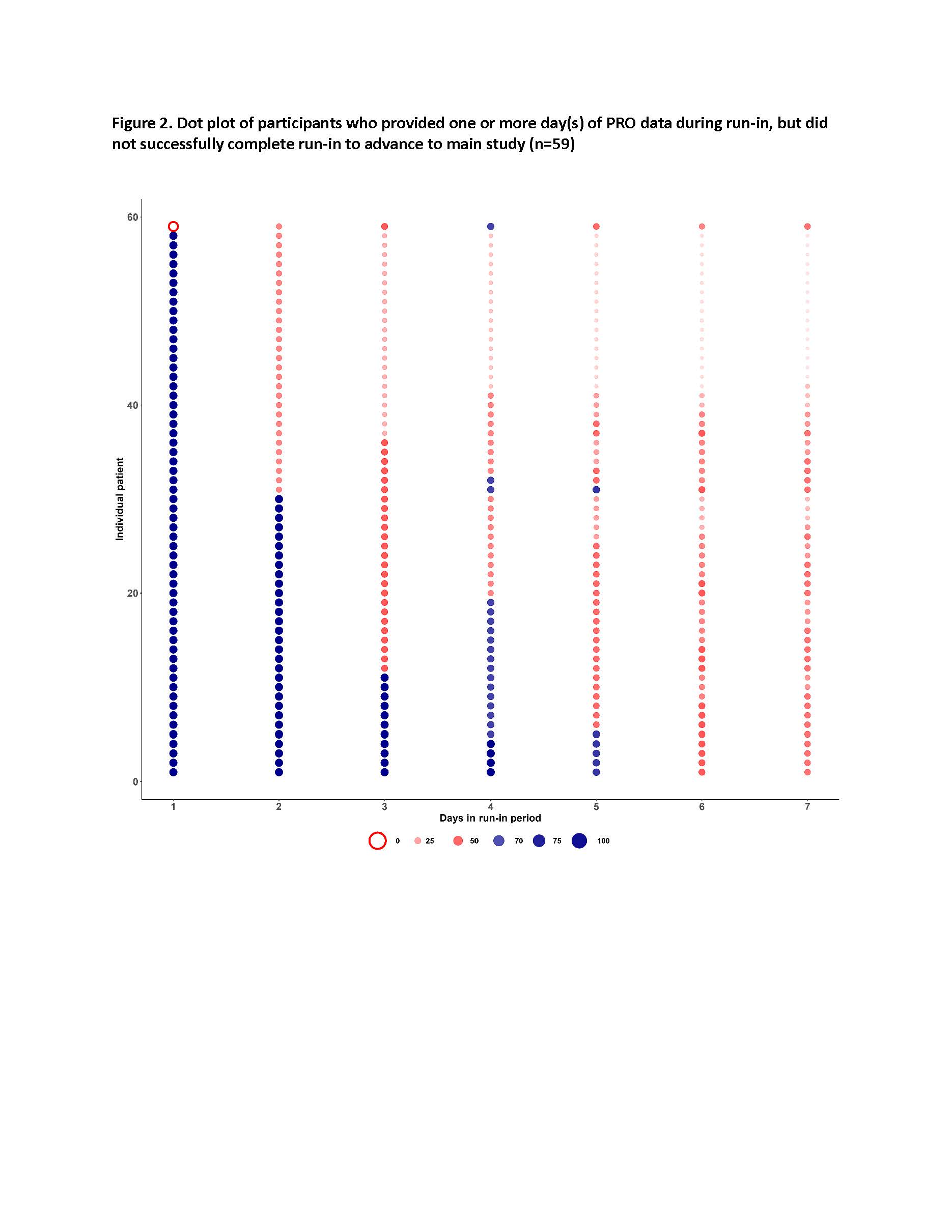
Results: A total of 299 patients enrolled and started the run-in. Of these, 240 entered the main study and met all inclusion criteria; at the time of this analysis, 148 participants have successfully completed the main study, with 95 of those (64%) providing both wearable and PRO data. Participants with more pain, fatigue, and worse physical function were more likely to fail the run-in based on inadequate PRO data capture (Table 1). Among the 148 participants who completed the main study, at least once weekly PROs were provided for 85% of weeks (Figure 1), and 83% of participants provided at least once weekly PROs for >= 9 of the 12 main study weeks. Daily PRO data was provided for 55% of days (Figure 1), and 38% of participants provided daily PRO data for at least 70% of all study days. Depending on the day of the week, time taken to complete PRO assessments each day ranged from a median of 1.4 minutes (IQR: 1.1 min – 2.1 min) to 2.1 minutes (IQR: 1.6 min – 3.2 min) among all participants. Among the participants who activated their wearable (n=95 to date), wearable data was provided for 74% of days for >= 70% of each 24-hour period (Figure 1), weekly PRO data was provided for 92% of week, daily PRO data was provided for 59% of days, and 38% of participants provided daily PRO data for at least 70% of all study days. Most patients who failed to meet minimum requirements to successfully complete the run-in did so within 3-4 days of starting the run-in (Figure 2).
Conclusion: Collecting weekly PRO data and daily wearable data for patients on RA treatment is feasible, although requiring daily PROs may be too burdensome in the context of routine care. Building some redundancy into the schedule of patient-generated data capture in order to optimize observations of disease activity and symptom changes over time as part of real-world evidence generation or remote therapeutic monitoring programs is advisable.
Abbreviations: RA, rheumatoid arthritis; DMARDs, disease-modifying antirheumatic drugs; bDMARDs, biologics or biologic DMARDS; csDMARDs, conventional synthetic DMARDs; tsDMARDs, targeted synthetic DMARDs; NRS, numeric rating scale; PROMIS, patient-reported outcomes measurement system; RADAI, rheumatoid arthritis disease activity index; CDAI, clinical disease activity index
PRO = patient-reported outcome
Representative rates of cumulative adherence to data submission show that individuals who did not progress to the main study had a marked drop in adherence as early as day 2. Dot size increases with increasing rate of adherence as shown and at 70% adherence, color of dot changes from red (<70% adherence) to blue (>70% adherence). Each dot in each column represents one participant who did not successfully complete the run-in and advance to the main study (n=59).
To cite this abstract in AMA style:
Nowell W, Clinton C, Xie F, Su Y, Stradford L, Curtis D, Zueger P, Patel P, Gavigan K, Venkatachalam S, Rivera E, Curtis J. Patient Engagement and Adherence to Digital Study Tasks: WEARable Activity Tracker Study Exploring Rheumatoid Arthritis Patients’ Disease Activity Using Patient-Reported Outcome Measures, Clinical Measures, and Biometric Sensor Data (the WEAR Study) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/patient-engagement-and-adherence-to-digital-study-tasks-wearable-activity-tracker-study-exploring-rheumatoid-arthritis-patients-disease-activity-using-patient-reported-outcome-measures-clin/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-engagement-and-adherence-to-digital-study-tasks-wearable-activity-tracker-study-exploring-rheumatoid-arthritis-patients-disease-activity-using-patient-reported-outcome-measures-clin/