Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. We explored characteristics of patients (pts) who discontinued (d/c) open-label, long-term extension (LTE) studies.
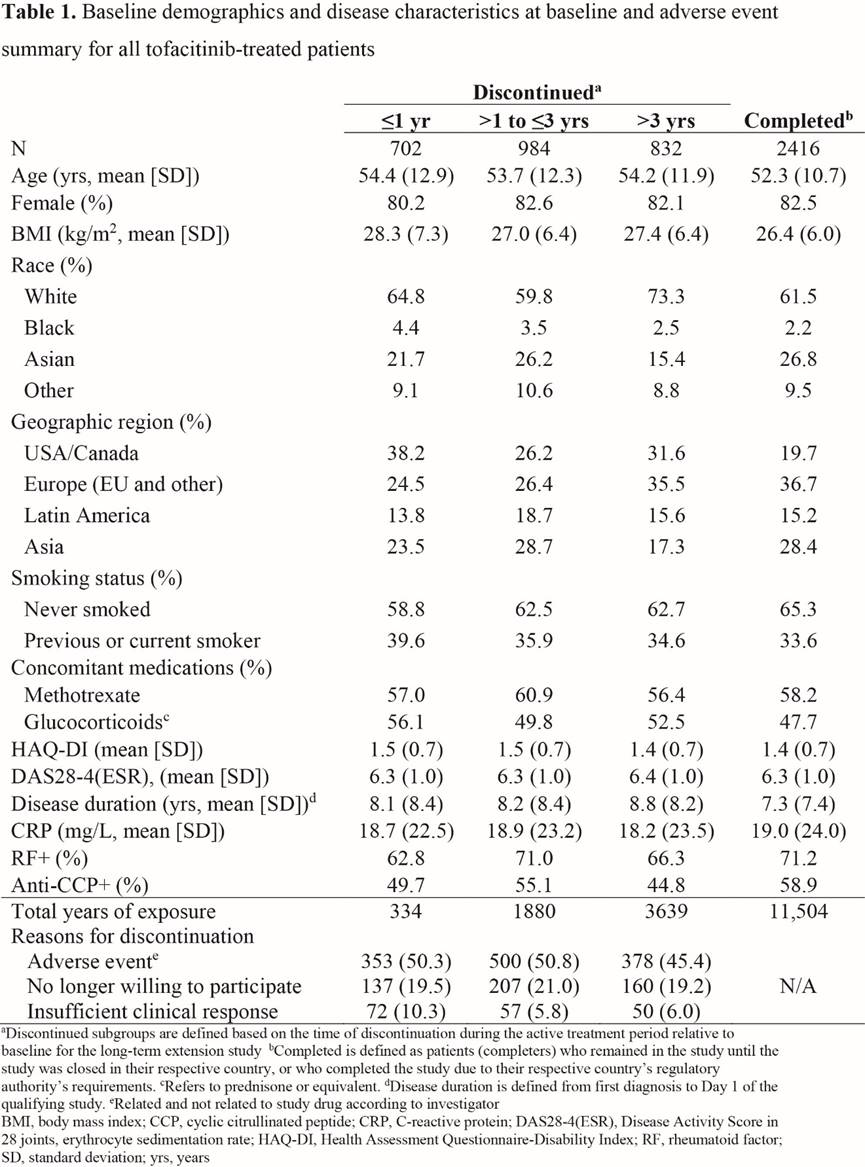
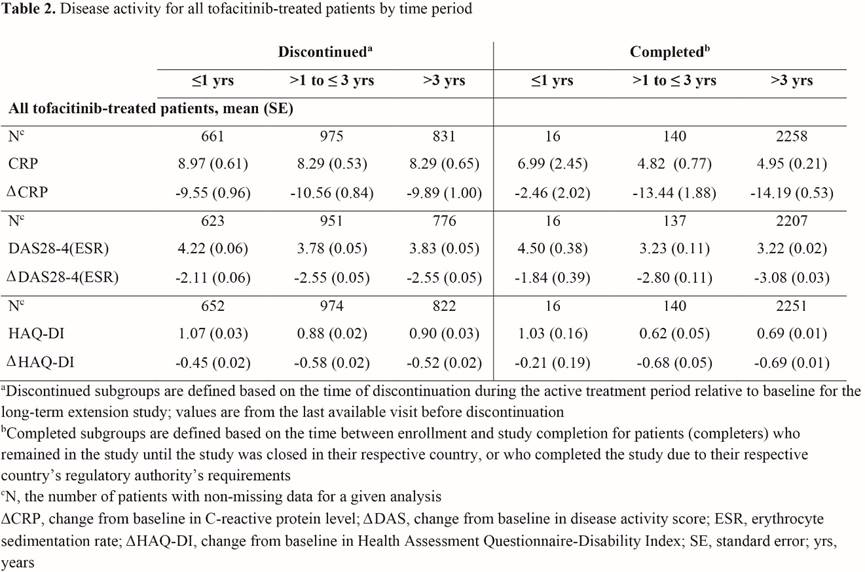
Methods: Data were pooled from 2 LTE studies (NCT00413699 [ORAL Sequel LTE main study database locked at time of analysis: March 2017] and NCT00661661) in pts with RA who participated in qualifying Phase 1/2/3 index studies. Pts (N=4967) received tofacitinib 5 or 10 mg BID as monotherapy or with conventional synthetic DMARDs. We analyzed pts who d/c within: ≤1 yr, >1–≤3 yrs, and >3 yrs, and study completers (Table 1). BL was that of the index study if pts began LTE treatment within 14 days of completing index study. Otherwise, LTE BL values were used. Demographics, reasons for discontinuation, disease activity (DAS28-4[ESR]), physical function (HAQ-DI), serology (CRP, RF, anti-cyclic citrullinated peptide [CCP]), concomitant medications, and treatment-emergent adverse events (TEAEs) were analyzed descriptively for all tofacitinib-treated pts.
Results: Of 4934 pts in this analysis, 2518 (51.0%) d/c (702 within <1 yr; 984 >1–≤3 yrs; 832 >3 yrs), predominantly due to AEs (n=1231; 48.9%), and 2416 (49.0%) pts were completers (Table 1); 33 pts ongoing in the LTE at database lock were not included. At BL, pts who d/c had numerically longer disease duration and more pts were from USA/Canada, current or ex-smokers, or used glucocorticoids than completers. Pts who d/c >1 yr had numerically lower values and a greater change from BL in CRP, DAS28-4(ESR), and HAQ-DI than pts who d/c ≤1 yr, and had higher values and a lower change from BL than completers for all parameters (Table 2). TEAEs were reported by 91.4% of evaluable completers and 80.6%, 92.0%, and 97.0% of evaluable pts who d/c within ≤1, >1–≤3, and >3 yrs, respectively. Of pts with TEAEs, 62.4%, 55.2%, and 46.8% d/c due to TEAEs within ≤1, >1–≤3, and >3 yrs, respectively. Among the most common TEAEs experienced by all groups were nasopharyngitis, upper respiratory tract infection, urinary tract infection, herpes zoster, and bronchitis. Pneumonia was more frequently observed in pts who d/c (5.7% [≤1 yr], 7.1% [>1–≤3 yrs], and 6.9% [>3 yrs]) than in completers (2.9%).
Conclusion: Pts who d/c had a longer disease duration at BL, and were more likely to use glucocorticoids, be smokers/ex-smokers, and be from USA/Canada than completers. Pts who d/c ≤1 year had higher disease activity than those who d/c >1 year; pts who d/c >1 year had higher disease activity than completers, supporting close monitoring of pts. Of pts with TEAEs, discontinuation due to TEAEs was more frequently observed in pts who d/c ≤1 yr.
To cite this abstract in AMA style:
Curtis JR, Wollenhaupt J, Chatzidionysiou K, Tas SW, Wang L, Shi H, Montoro M, Neregård P, Dahl P, Tsekouras V. Patient Characteristics Associated with Discontinuation of Tofacitinib for the Treatment of Rheumatoid Arthritis in Open-Label, Long-Term Extension Studies up to 9.5 Years [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/patient-characteristics-associated-with-discontinuation-of-tofacitinib-for-the-treatment-of-rheumatoid-arthritis-in-open-label-long-term-extension-studies-up-to-9-5-years/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-characteristics-associated-with-discontinuation-of-tofacitinib-for-the-treatment-of-rheumatoid-arthritis-in-open-label-long-term-extension-studies-up-to-9-5-years/