Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
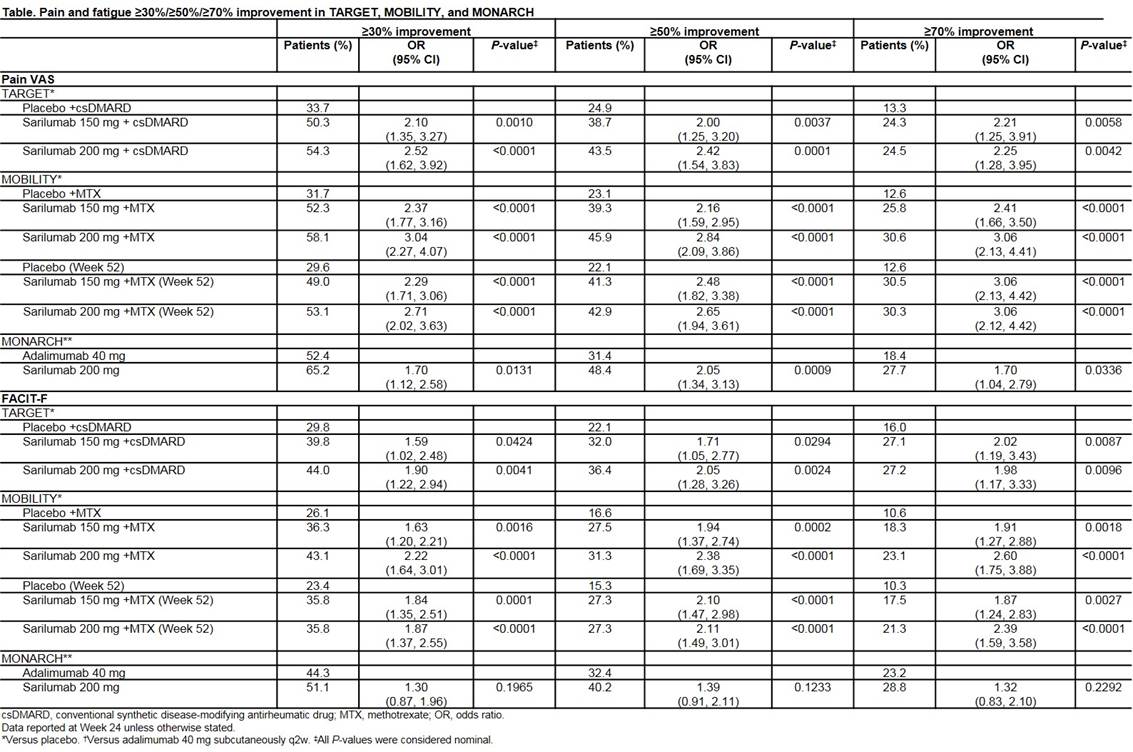
Background/Purpose: Pain and fatigue are common symptoms of rheumatoid arthritis (RA) and can severely impact patients’ quality of life. With multiple biologic disease-modifying antirheumatic drugs (bDMARDs) available for RA, symptoms such as pain and fatigue may play an increasing role in shared decision-making. Sarilumab is a human monoclonal antibody that blocks interleukin-6 (IL-6) from binding to both membrane-bound and soluble IL-6 receptor-α; it is indicated for the treatment of moderate-to-severely active RA in adult patients who have had an inadequate response to ≥1 DMARDs. The objective of this study was to explore the effect of sarilumab on pain and fatigue in patients with moderate-to-severely active RA.
Methods: Post hoc statistical analyses were performed using data from sarilumab randomized controlled trials: TARGET (NCT01709578) and MOBILITY (NCT01061736) (sarilumab 150 or 200 mg every 2 weeks [q2w] vs placebo, combined with conventional synthetic DMARDs), and MONARCH (NCT02332590), (sarilumab 200 mg q2w vs adalimumab 40 mg q2w). At each study visit, pain was assessed using a 0–100 mm visual analog scale (VAS); fatigue was assessed using the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale. The proportion of patients achieving ≥30%/≥50%/≥70% pain VAS and FACIT-F improvement, and median time to first pain VAS and FACIT-F ≥50% improvement was assessed at Week 24 in TARGET and MONARCH, and Weeks 24 and 52 in MOBILITY (not pooled). Median time to first pain VAS and FACIT-F ≥50% improvement was analyzed by Kaplan–Meier. P-values were considered nominal.
Results: 546 patients from TARGET, 1197 from MOBILITY, and 369 from MONARCH were included. In TARGET and MOBILITY, more patients receiving sarilumab 150 or 200 mg achieved ≥30%/≥50%/≥70% improvements in pain and fatigue than placebo. In MONARCH, more patients receiving sarilumab 200 mg achieved ≥30%/≥50%/≥70% improvements in pain and fatigue than adalimumab. (Table). Overall, pain was improved by at least 30% in around 50% of patients and fatigue in 40% of patients (Table). Median time to first pain ≥50% improvement was shorter for patients receiving sarilumab 200 mg than both placebo (12 vs 24 weeks) in TARGET and MOBILITY or adalimumab (12 vs 16 weeks) in MONARCH. Median time to first fatigue ≥50% improvement was shorter for patients receiving sarilumab 200 mg vs placebo (TARGET: 12 vs 24 weeks, MOBILITY 52 weeks vs not reached) with no significant advantage vs adalimumab. In these clinical studies, sarilumab had a safety profile consistent with IL-6 inhibition.
Conclusion: In this study of patients with moderate-to-severely active RA, sarilumab demonstrated faster and greater pain improvement vs placebo or adalimumab. In addition, sarilumab demonstrated faster and greater fatigue improvement vs placebo but not adalimumab. These results are important to consider in RA treatment decision-making.
To cite this abstract in AMA style:
Gossec L, Boklage S, St. John G, van Hoogstraten H, Kimura T. Pain Is Improved in Around 50% of Patients and Fatigue in 40% of Patients with Rheumatoid Arthritis Treated with Sarilumab in the Target, Mobility and Monarch Trials [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/pain-is-improved-in-around-50-of-patients-and-fatigue-in-40-of-patients-with-rheumatoid-arthritis-treated-with-sarilumab-in-the-target-mobility-and-monarch-trials/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-is-improved-in-around-50-of-patients-and-fatigue-in-40-of-patients-with-rheumatoid-arthritis-treated-with-sarilumab-in-the-target-mobility-and-monarch-trials/