Session Information
Date: Monday, November 8, 2021
Title: RA – Treatments Poster II: PROs, Biomarkers, & Systemic Inflammation (1223–1256)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Baricitinib (BARI) is a Janus kinase (JAK)1/JAK2 inhibitor which provides improvements to clinical signs, symptoms, and patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA), with a notable effect on pain.1,2,3 Pain in RA is multifactorial, with inflammation being an important, but not exclusive, cause.
Methods: Data for these analyses were derived from the Phase 3 study RA-BEAM (NCT01710358). Changes in pain from baseline to week (W)12 and W24 were evaluated using a 0-100 mm visual analog scale. To assess the relationship between pain and inflammation, results were compared between patients receiving BARI 4-mg + MTX and those receiving adalimumab (ADA) + MTX in both patients who achieved ≥ 50% improvement in swollen joint count (SJC) and those who did not at W12 and W24. At each visit, the analysis of covariance (ANCOVA) model was applied which includes the change from baseline of pain as the response variable and baseline pain, region, baseline joint erosion status, treatment, SJC 50% improvement subgroup and the interaction of treatment and subgroup as the explanatory variables. P-values were calculated for both treatment comparisons and the interaction term and were not adjusted for multiplicity.
Results: Consistent with previously reported clinical efficacy, most patients achieved SJC 50% improvement when treated with BARI + MTX (365/487, 74.9%) and ADA + MTX (228/330, 69.1%) at W12. For patients who did not achieve 50% improvement in SJC at W12, those treated with BARI 4-mg + MTX reported significantly greater improvements in pain (-24.3 vs -13.4, p< 0.001) than ADA + MTX (Fig 1A). This difference was not observed consistently in the group achieving SJC 50% (Fig 1B). The significant interaction test (p=0.008) between treatment and subgroup defined by SJC 50% improvement at W12 implies a differential effect of BARI on pain not associated with the improvement in inflammation. A similar trend was found at W24 (Fig 2). Additionally, at W12, the magnitude of pain reduction difference between BARI + MTX and ADA + MTX in the groups achieving or not achieving SJC 20% improvement was directionally similar to results using SJC 50% as the threshold, but this analysis is limited by small sample size in the non-responder group.
Conclusion: BARI 4-mg + MTX treatment provided superior pain relief compared to ADA + MTX in patients with RA who did not achieve ≥ 50% improvement in SJC at W12 and W24. These results may point toward a differential effect of BARI on pain mediated outside of the inflammatory pathway.
References
1. Taylor et al. N Engl J Med. 2017;376(7):652-662.
2. Keystone et al. Ann Rheum Dis. 2017;76(11):1853-1861.
3. Taylor et al. J Clin Med. 2019;8(6).
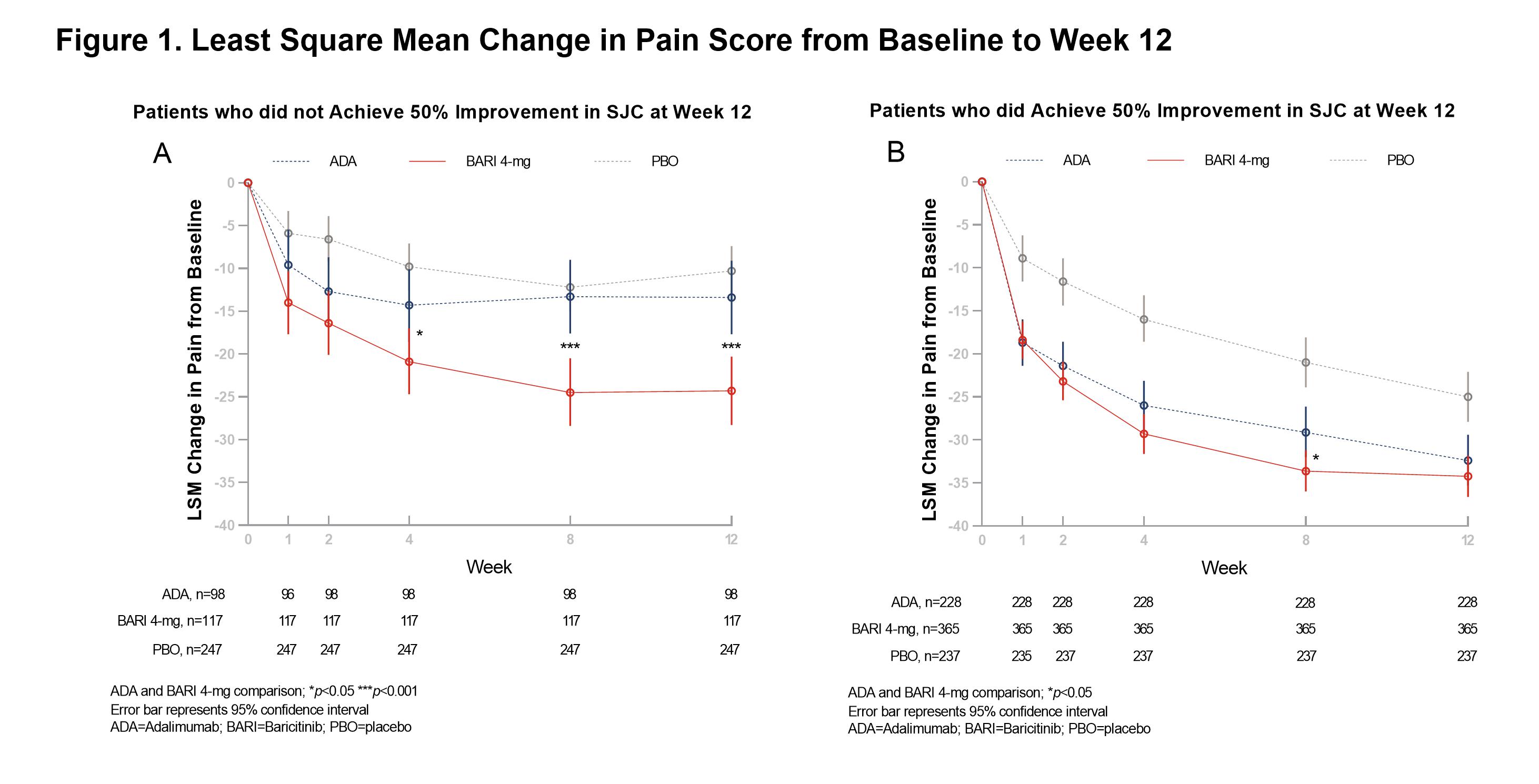 Figure 1. Least Square Mean Change in Pain Score from Baseline to Week 12
Figure 1. Least Square Mean Change in Pain Score from Baseline to Week 12
 Figure 2. Least Square Mean Change in Pain Score from Baseline to Week 24.
Figure 2. Least Square Mean Change in Pain Score from Baseline to Week 24.
To cite this abstract in AMA style:
Sebba A, Wang D, Jia B, Troutt J, Birt J, Quebe A, Taylor P. Pain in Patients with Rheumatoid Arthritis Who Did or Did Not Achieve Treatment Response Based on Improvement in Swollen Joints with Baricitinib Clinical Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/pain-in-patients-with-rheumatoid-arthritis-who-did-or-did-not-achieve-treatment-response-based-on-improvement-in-swollen-joints-with-baricitinib-clinical-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pain-in-patients-with-rheumatoid-arthritis-who-did-or-did-not-achieve-treatment-response-based-on-improvement-in-swollen-joints-with-baricitinib-clinical-trials/
