Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: RA is an autoimmune disease affecting over 1.5 million Americans. Biologic disease-modifying antirheumatic drugs (bDMARDs) expanded treatment choices for RA patients (pts) in the last two decades with a significant impact on cost of care. As RA treatment evolves, there is a need for analysis of treatment costs in the modern era, particularly to inform new payment methodologies. As the majority of RA care in the US occurs in the community setting, we evaluated RA-related treatment costs in a community practice cohort.
Methods: Using electronic medical records from 8 large mid-Atlantic rheumatology practices, adult pts with RA diagnosis (International Classification of Diseases 9th revision (ICD- 9) 714, ICD-10 M05, M06) who initiated or switched to a new bDMARD during April 2016-March 2018 were selected. We accounted for cost of care provided by the rheumatology practices including E&M, DMARDs, bDMARDs, steroids, other services including drug administration and labs. Pts were followed for 12 months from initiation of biologic or switch. Annual costs were standardized to 2019 USD using Centers for Medicare & Medicaid Services Average Sales Price, Part D Medication Price, Physician, and Clinical Laboratory Fee Schedules. Comorbidities (comorbids) were assigned based on pt reported medications. Differences between pts with comorbids vs. without were assessed using t-test for continuous variables and chi-square test for categorical variables; costs were also assessed using propensity score matching (PSM) and generalized linear models (GLM) with gamma distribution and log link function to account for differences in pt characteristics.
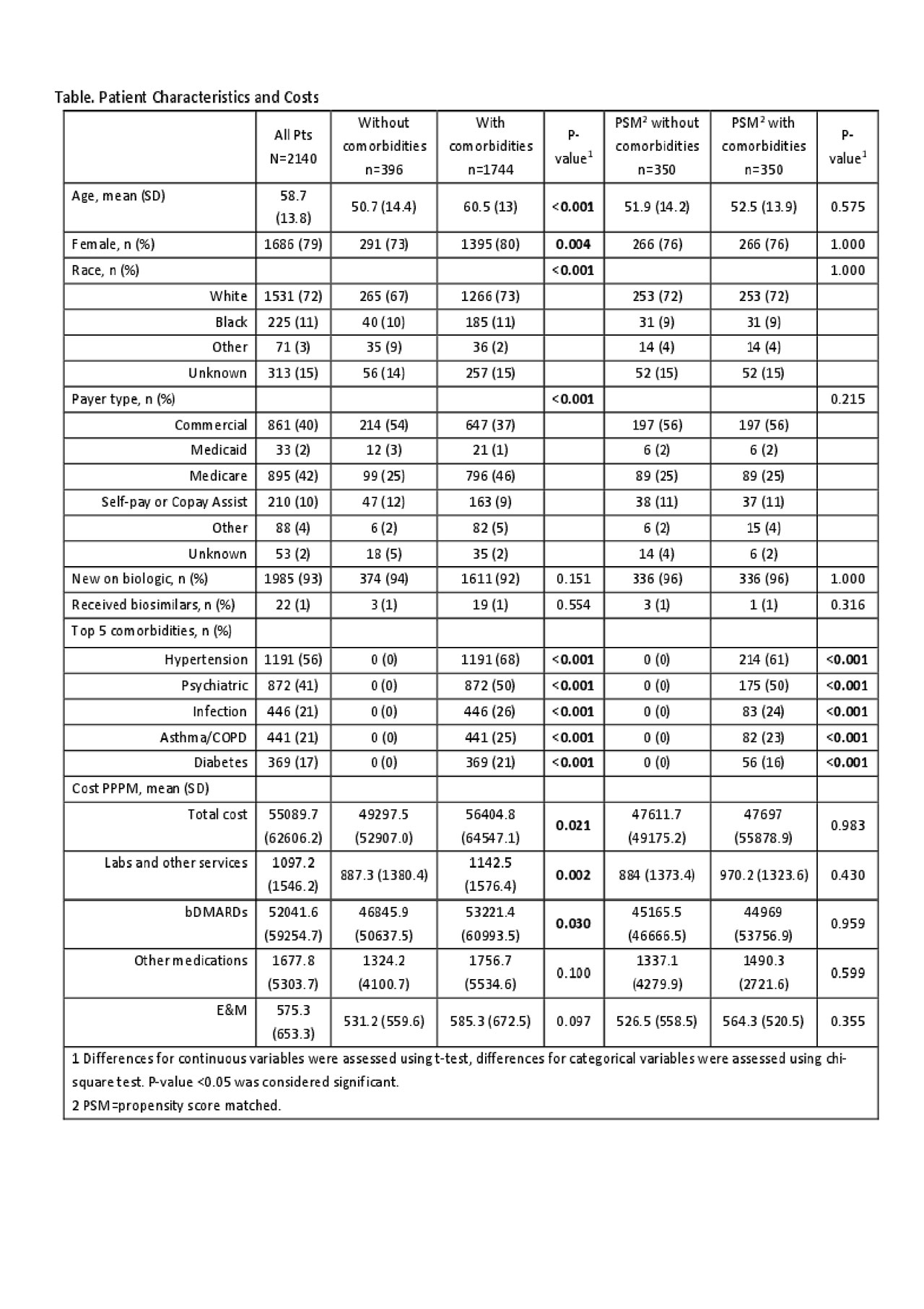
Results: Of 2140 pts, 1744 (82%) had at least 1 comorbid. Compared to pts without comorbids, pts with comorbids were older, more likely to be white, female, and Medicare-insured (Table). Before accounting for differences in demographics, pts with comorbids had significantly higher costs of bDMARDs, labs/other services, and total costs. After accounting for differences in demographics using GLM, pts with comorbids had higher E&M costs ($569 (CI: 524-618) vs. $519 (CI: 471-573), p=0.027) but no difference in costs of bDMARDs, labs, other medications, and total costs. After PSM there were no differences in age, race, insurance, and gender between cohorts and 350 pts per group matched without replacement, representing 20% of pts with comorbids and 88% of pts without comorbids. Among PSM pts, there were no differences in annual costs between cohorts. Prescription of the commercially available biosimilars was found low (1%) during the study period, thus their impact on costs was not evaluated.
Conclusion: Among RA pts treated with bDMARDs in the community from 2016 to 2018, the majority of the pts had comorbids. Pts with comorbids had significant differences in their characteristics compared to those without comorbids and incurred higher RA-related annual costs, however when accounting for differences in pt characteristics, annual costs of RA-related care were not different between the groups. This initial analysis suggests that comorbids may not be driving costs of RA-related care as it has been presumed. Further research is warranted to evaluate the impact of individual comorbids.
To cite this abstract in AMA style:
Edgerton C, Radtchenko J, Holers V. Outpatient Costs and Evaluation and Management (E&M) Expenditure Trends in Rheumatoid Arthritis (RA) Patients Treated with Biologic Therapies in US Community Practices [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/outpatient-costs-and-evaluation-and-management-em-expenditure-trends-in-rheumatoid-arthritis-ra-patients-treated-with-biologic-therapies-in-us-community-practices/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outpatient-costs-and-evaluation-and-management-em-expenditure-trends-in-rheumatoid-arthritis-ra-patients-treated-with-biologic-therapies-in-us-community-practices/