Session Information
Date: Monday, November 8, 2021
Title: Epidemiology & Public Health Poster III: Other Rheumatic & Musculoskeletal Diseases (1022–1060)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is used to treat rheumatoid arthritis (RA), connective tissue disease (CTD) and other inflammatory conditions. In 2018, the United Kingdom Royal College of Ophthalmologists (RCOphth) published new screening guidelines to monitor the risk of HCQ associated retinopathy, recommending patients be referred for screening if patients had been on HCQ for ≥5 years and included a list of risk factors to guide the screening process: dosage >5mg/kg/day absolute body weight (ABW), concurrent tamoxifen use and impaired renal function (eGFR < 60ml/min/1.73m²). This report aims to audit the 1) HCQ dosing based on RCOphth 2018 guidelines 2) the number of patients appropriately referred for screening and 3) the outcome of screening.
Methods: In July 2018 (when the RCOphth guidelines were published), a list of all patients started on HCQ from April 2013-2014 and received the first prescription from hospital was obtained from hospital pharmacy records. These patients’ electronic patient records were reviewed between Jul-Oct 2018 to extract data on demographic and clinical parameters, drug persistence including reason for cessation of drug and suitability for referral for HCQ retinopathy monitoring if they continued the drug. Referral for eye screening took place between Oct 2018-May 2019. These patients were followed until the most recent follow-up visit by May 2021 to determine the outcome of screening (Figure 1).
Results: 100 patients were identified. The median age of the cohort was 57 years (46-70), with 67% of the cohort being female. Many patients (67%) were started on HCQ for RA, 16% for SLE and 17% for other rheumatological diagnoses. The median HCQ dose was 4.6mg/kg/day (3.4-5.7). 37% of patients were prescribed a starting dose of > 5mg/kg/day. 9 patients had an eGFR of < 60 ml/min/1.73m2. No patients were on concurrent tamoxifen (Table 1).
By July 2018 (median 5 years of follow-up from 2013), 48% of patients remained on HCQ. 52% of patients were no longer on HCQ for a variety of reasons including side effects (33%) and ineffectiveness (10%). Of the 48 patients that remained on HCQ, 3 died before they could be referred and the remaining 45 were referred for eye screening. Of the 45 who were referred, 22 were screened and all showed no evidence of retinopathy, 14 referrals were lost, 6 did not attend and were discharged, 2 cancelled and did not rebook and 1 referral was returned due to the COVID-19 outbreak (Figure 1). Of the 22 patients with normal screening, 2 had renal impairment and 7 were prescribed ≥5mg/kg/day and 2 had both renal impairment and were dosed over 5mg/kg.
Conclusion: After 5 years of follow-up, the drug survival for HCQ is 50%. By knowing the number of patients prescribed and started on HCQ annually and stop therapy after 5 years, this will influence screening service capacity issues. Of those patients who attended for screening after 5 years of therapy, none had evidence of HCQ retinopathy which is reassuring including 11 patients who were either dosed ≥5mg/kg/day (n=7) or had renal impairment (n=2) or both (n=2). In 2013, over a third of patients were started on a HCQ dose >5mg/kg/day, we need to ensure that dosage is appropriate and monitor for other factors to reduce their risk of developing HCQ retinopathy.
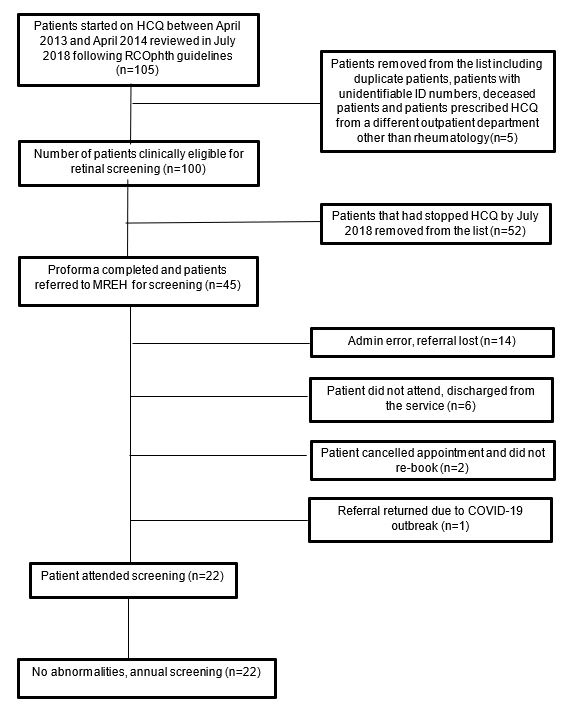 Figure 1: Flowchart showing the process of filtering patients who were clinically suitable to be referred for HCQ retinopathy screening and the outcome of screening (HCQ=hydroxychloroquine).
Figure 1: Flowchart showing the process of filtering patients who were clinically suitable to be referred for HCQ retinopathy screening and the outcome of screening (HCQ=hydroxychloroquine).
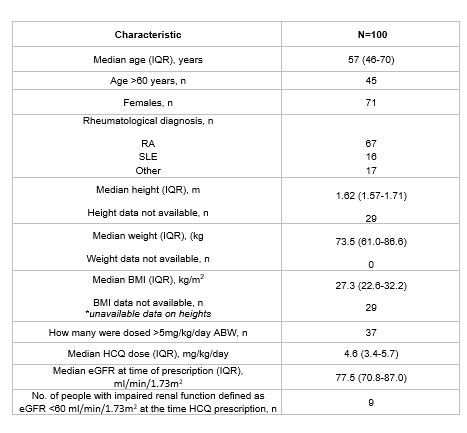 Table 1- Baseline characteristics of cohort that started HCQ between April 2013_2014 (ABW = absolute body weight, HCQ=Hydroxychloroquine, IQR= interquartile range, RA=Rheumatoid Arthritis, SLE= Systemic Lupus Erythematous, BMI= Body Mass Index, eGFR= estimated Glomerular Filtration Rate).
Table 1- Baseline characteristics of cohort that started HCQ between April 2013_2014 (ABW = absolute body weight, HCQ=Hydroxychloroquine, IQR= interquartile range, RA=Rheumatoid Arthritis, SLE= Systemic Lupus Erythematous, BMI= Body Mass Index, eGFR= estimated Glomerular Filtration Rate).
To cite this abstract in AMA style:
Tehseen G, Chadwick A, Wills S, Low A. Outcomes of Hydroxychloroquine Screening for Retinopathy in a Cohort of Patients with Rheumatological Conditions [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/outcomes-of-hydroxychloroquine-screening-for-retinopathy-in-a-cohort-of-patients-with-rheumatological-conditions/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-of-hydroxychloroquine-screening-for-retinopathy-in-a-cohort-of-patients-with-rheumatological-conditions/
