Session Information
Date: Sunday, November 7, 2021
Title: Measures & Measurement of Healthcare Quality Poster (0623–0659)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Experience with rituximab (RTX) in autoimmune rheumatic disease (AIRD) has shown a clear association with hypogammaglobulinemia, serious infections, and impaired humoral response to certain vaccines. The 2019 EULAR recommendations provide guidance to time future vaccines at least 24 weeks post-RTX and 4 weeks before the next course. We sought to educate faculty on the rationale for timing SARS-CoV-2 vaccines in AIRD patients on RTX and to assess timing of vaccination in clinical practice.
Methods: We performed a medical records review of AIRD patients treated with RTX from January 2020 through to February 2021 at our institution and conducted a quality improvement study to optimize vaccine timing for patients with AIRD on RTX. We first educated providers on the rationale for timing SARS-CoV-2 vaccines 24 weeks post RTX and recorded provider vaccine preference to either universally time all vaccines or to individualize the plan for each patient. We measured actual timing of vaccines through telephone survey of patients. In patients with available data, we then analyzed vaccine response in relation to vaccine timing and other clinical and immunologic parameters. This project was determined to be exempt by the institutional IRB.
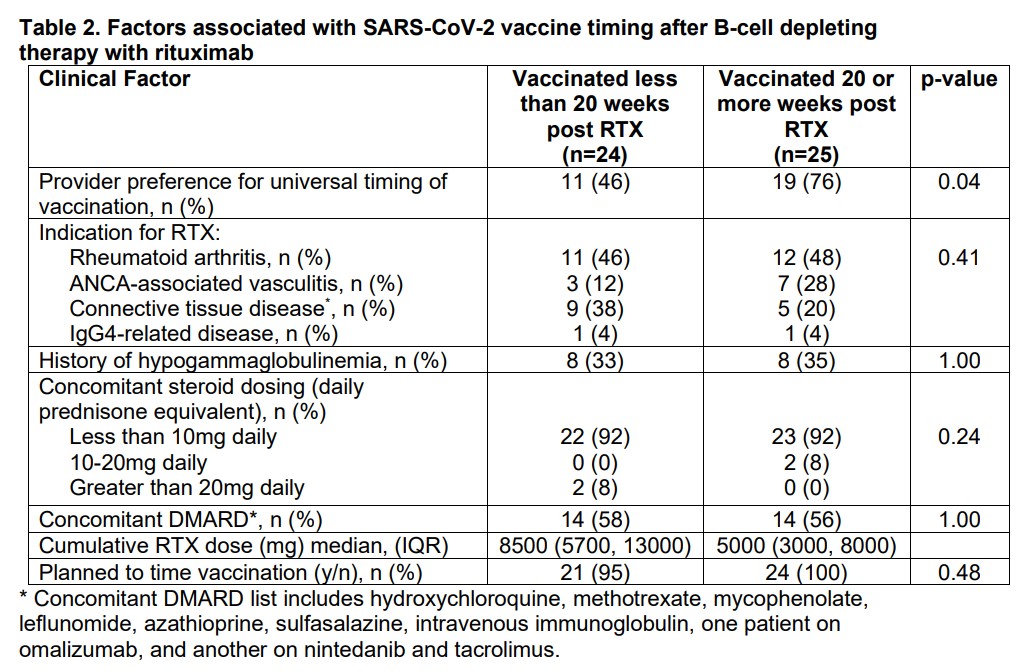
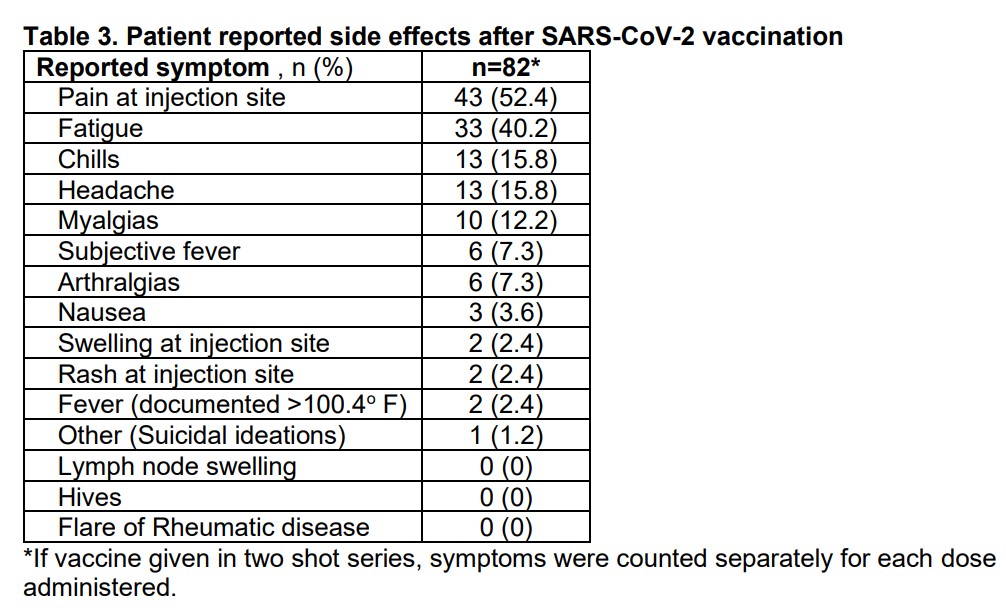
Results: A total of 91 patients received RTX for AIRD during the COVID-19 pandemic. Seventy patients had plans to continue RTX at our institution in 2021. Of the 49 vaccinated patients, the median time of vaccination post RTX was 20.14 (12.4, 25.7) weeks with 14 (28.6%) patients vaccinated at least 24 weeks and 25 (51%) at least 20 weeks post RTX. Patients whose providers expressed universal intent to time vaccines were more likely to be vaccinated at least 20 weeks post-RTX than patients whose providers took an individualized approach to vaccine timing (p= 0.04). All patients who received Moderna or Pfizer vaccines received a second dose 2-7 weeks later. A higher cumulative RTX dose was associated with vaccination less than 20 weeks post-RTX (p= 0.03). Preliminary results for the 9 patients with SARS CoV2 Spike protein IgG Ab’s revealed negative titers in the majority (78%) of patients despite a median time to vaccination post of 22.1 (19.7, 22.8) weeks.
Conclusion: While a majority of patients received dose 1 of the SARS-CoV2 vaccine more than 20 weeks after their last RTX infusion; most patients did not meet the primary endpoint of vaccination 24 weeks post-RTX despite a physician-directed educational program advocating for this. Patients of providers who indicated a preference to universally delay vaccination were more likely to receive their first vaccine dose at least 20 weeks post-RTX. Preliminary results are concerning for a low rate of SARS-CoV2 spike IgG Ab development in patients despite optimal vaccine timing and low steroid doses. These findings are of particular concern given emerging signals that RTX may be associated with particularly severe COVID-19 infections and warrant further study.
To cite this abstract in AMA style:
Magliulo D, Wade S, Kyttaris V. Optimizing SARS-CoV-2 Vaccine Timing in Rituximab-Treated Patients with Autoimmune Rheumatic Diseases: A Quality Improvement Intervention [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/optimizing-sars-cov-2-vaccine-timing-in-rituximab-treated-patients-with-autoimmune-rheumatic-diseases-a-quality-improvement-intervention/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimizing-sars-cov-2-vaccine-timing-in-rituximab-treated-patients-with-autoimmune-rheumatic-diseases-a-quality-improvement-intervention/