Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
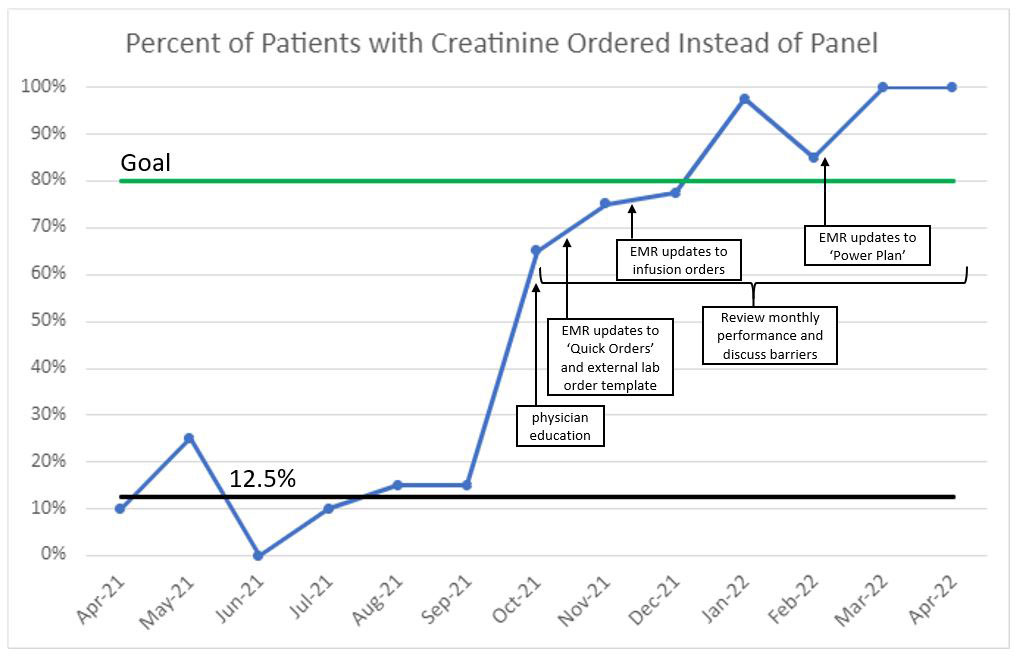
Background/Purpose: Most children with juvenile idiopathic arthritis (JIA) are treated with medications that require medication safety monitoring labs. Recommended laboratory testing includes a creatinine level. However, 87.5% of our baseline visits had a basic metabolic panel (BMP) ordered instead of a creatinine alone. The difference in cost for ordering a serum creatinine versus a BMP is $31. The monetary difference in matched net revenue per unit, a better estimate of healthcare cost, is $12.09. This leads to increased healthcare costs to the hospital, payers, and families. Our project aim was to increase the ordering of a creatinine rather than a BMP in patients with non-systemic JIA needing methotrexate, leflunomide, and/or biologic medication safety lab monitoring in the Rheumatology Clinic from 12.5% to 80% in 8 months.
Methods: Education was provided to physicians on medication monitoring recommendations and cost differences between ordering a creatinine versus a BMP. We created process maps to analyze all the different ways that our patients get labs done. We incorporated this project in our pre-visit planning process. Our primary interventions involved updates to our electronic medical record (EMR): 1) addition of creatinine order to our ‘Favorites’, ‘Quick Orders’, and ‘Power Plan’ for internal orders; 2) addition of creatinine to our external laboratory order template; and 3) addition of creatinine to our infusion order sets. We did a sampling of 40 patients each month to collect data on our process measure, which was the percent of patients with JIA taking methotrexate, leflunomide, TNF inhibitors, abatacept, and/or tocilizumab who had a creatinine alone checked rather than a BMP for medication monitoring safety laboratory tests and created a run chart to review and track our performance over time.
Results: Our 6-months of baseline data from April to September 2021 had a median of 12.5% of visits having a creatinine alone checked instead of a BMP (Figure 1). Data from October to December 2021 were 65-78%, and we have consistently surpassed our goal of greater than 80% of patients having creatinine ordered since January 2022. There were no unintended consequences noted during our project.
Conclusion: Our project optimized laboratory medication safety monitoring in JIA patients by eliminating unnecessary tests to save costs and advance value-based care. Our primary interventions of education and EMR modifications allowed for standardization of laboratory test ordering. Some physicians in our group have expanded this project with other changes to their laboratory test ordering practice that could lead to decreased cost without sacrificing patient safety.
To cite this abstract in AMA style:
Harris J, Favier L, Fox E, Holland M, Hoffart C, Ibarra M, Jones J, Harris L, Cooper A. Optimizing Laboratory Medication Safety Monitoring in Patients with JIA to Advance Value-based Care [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/optimizing-laboratory-medication-safety-monitoring-in-patients-with-jia-to-advance-value-based-care/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimizing-laboratory-medication-safety-monitoring-in-patients-with-jia-to-advance-value-based-care/