Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The emergence of biological disease modifying drugs (bDMARD) has allowed a targeted approach to rheumatoid arthritis (RA) (“treat-to-treat” strategy). Once sustained remission is achieved, optimized treatment of bDMARD (OT) is considered: using drugs at lower doses than indicated in data sheets. Studies show that 33-64.2% of patients on OT lose remission in the first 6 months. Still, it is a feasible practice in selected patients and so, it is of interest to study the situation of optimized patients to improve our practice. Our objectives were to describe demographic, clinical, analytical and therapeutic characteristics of RA patients on OT in our hospital. Secondly, to study the survival of OT and to compare patients with survival longer or shorter to one year.
Methods: We did a retrospective review of the medical records of RA patients who began bDMARD OT [Abatacept (ABA), anti-TNF drugs and Tocilizumab (TCZ)] between January 2014 and December 2018. We defined the end of OT as the restart of the usual dose. Continuous variables are described with mean and standard deviation (SD) and qualitative variables are shown in absolute value and percentage. We divided the sample into patients with OT survival greater than or equal to one year and patients with OT survival less than one year, after which the characteristics of both populations were compared. Categorical variables were analyzed using Pearsons chi2 and quantitative variables using Student’s t-test. Survival analysis was performed using a Kaplan-Meier estimator.
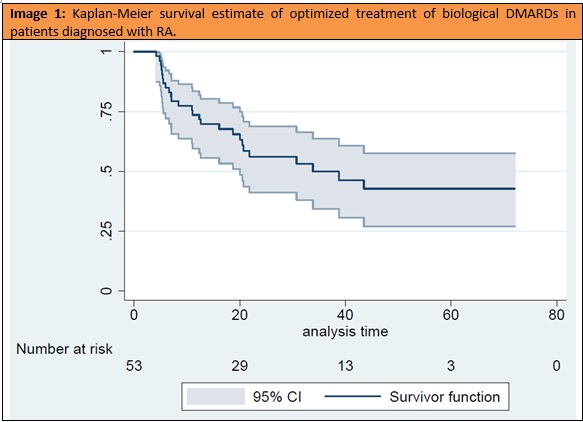
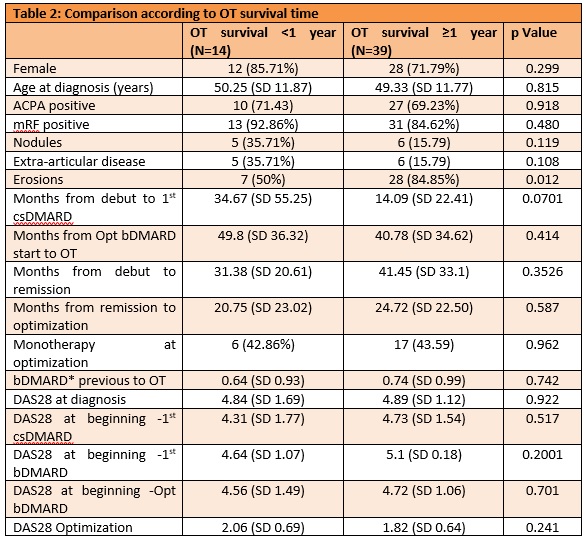
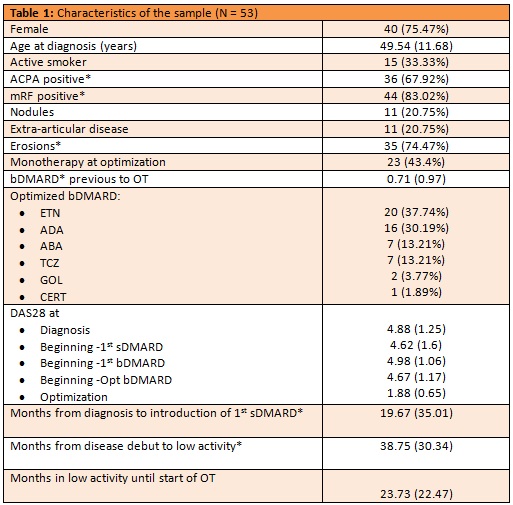
Results: We identified 234 RA patients on bDMARD at our hospital, of which 53 (22.6%) had been optimized between January 2014 and December 2018: 39 (73.6%) with anti-TNF, 7 (13.2) with ABA and 7 (13.2%) with TCZ. Their characteristics are shown in table 1. It is worth mentioning the rate of monotherapy (43.3%) and the low number of bDMARD prior to optimization (median 0.71, SD 0.97). The median survival of OT was 33.8 months (Image 1) and thirty-nine patients (73.6%) maintained OT for at least one year (95% confidence interval, 0.59 to 0.83). When comparing patients with survival greater/equal versus shorter to one year (table 2), the only variable showing significant differences was the presence of erosions at beginning of OT (28 patients in the >1 year group vs 7 in the < 1 year group; p=0.012). Although the difference is not significant (p = 0.07), patients with a survival of less than one year have more time between the debut and the beginning of the first conventional synthetic DMARD (csDMARD).
Conclusion: Two limitations should be noted: the sample size and the lack of a homogeneous optimization schedule among rheumatologists. More studies are needed to define the characteristics of patients who can safely benefit from OT.
 ACPA: anti-citrullinated protein antibodies; mRF: monoclonal rheumatoid factor; Erosions: presence of erosions at Optimization; bDMARD: biological DMARD; ETN: Etanercept; ADA: Adalimumab; ABA: Abatacept; TCZ: Tocilizumab; GOL: Golimumab; CERT: Certolizumab; Opt bDMARD: bDMARD optimized; csDMARD: conventional synthetic DMARD; Low activity: DAS28 < 3.2
ACPA: anti-citrullinated protein antibodies; mRF: monoclonal rheumatoid factor; Erosions: presence of erosions at Optimization; bDMARD: biological DMARD; ETN: Etanercept; ADA: Adalimumab; ABA: Abatacept; TCZ: Tocilizumab; GOL: Golimumab; CERT: Certolizumab; Opt bDMARD: bDMARD optimized; csDMARD: conventional synthetic DMARD; Low activity: DAS28 < 3.2
To cite this abstract in AMA style:
De Diego Sola A, Egües Dubuc C, Alcorta Lorenzo N, Valero Jaimes J, Maíz Alonso O, Lopez Dominguez L, Uriarte Isacelaya E, Cancio Fanlo J, Aranguren Redondo M, Irastorza Larburu M, Belzunegui Otano J. Optimized Treatment of Biological Disease Modifying Drugs in Routine Clinical Practice: Survival Study and Analysis of Patient Characteristics [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/optimized-treatment-of-biological-disease-modifying-drugs-in-routine-clinical-practice-survival-study-and-analysis-of-patient-characteristics/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/optimized-treatment-of-biological-disease-modifying-drugs-in-routine-clinical-practice-survival-study-and-analysis-of-patient-characteristics/