Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) have improved outcomes for many types of cancer, but the therapy is known to cause immune-related adverse events (IRAE). ICI can cause early or late onset inflammatory arthritis, which can continue after stopping ICI1,2. Many cases are negative for RF and ACPA. Timely recognition and treatment may improve symptoms and facilitate continuation of cancer therapy.
Methods: We reviewed the Loyola University Medical Center database to identify patients who had new-onset arthritis in the setting of ICI cancer therapy. We collected data on clinical and diagnostic features and outcome measures. Patients received at least one dose of ICI (atezolizumab, durvalumab, ipilimumab, nivolumab, pembrolizumab) between January 1, 2011 and October 31, 2019 and were identified on manual review by two rheumatologists. Definite inflammatory arthritis was based on the presence of documented diagnosis by a physician, exam, or imaging findings. Probable diagnosis was based on clinical description. Arthritis course was described as resolved or sustained, with sustained defined as at least 3 months of arthritis symptoms or treatment. Tumor response was categorized according to the Response Evaluation Criteria for Solid Tumors on oncology follow up. We used descriptive statistics, with frequency and percentage for categorical variables.
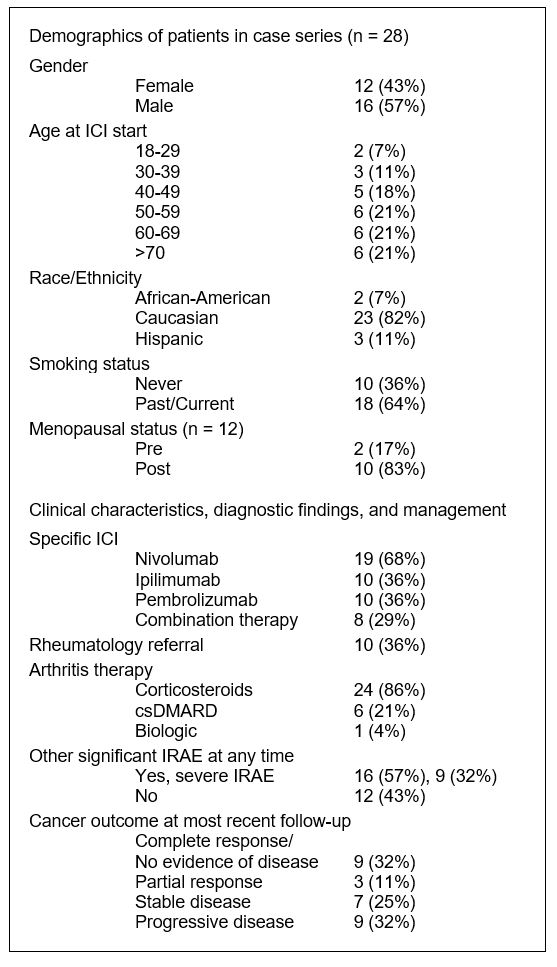
Results: A total of 801 patients received ICI. Among these, 28 patients were identified as having ICI-induced arthritis (Table 1, Fig. 1). Median age was 57.5 years. Most common tumor types were melanoma, renal, non-small cell lung, and bladder. Mean follow up time from ICI start was 24.3 months (range 4-62).
RF and ACPA were negative in all 5 patients for whom they were measured. One patient had a positive RF that pre-dated cancer therapy. 32% of patients had severe IRAE (hepatitis, hypophysitis, adrenal insufficiency, ITP, pancytopenia, severe colitis). Patients with a resolved course of arthritis were more likely to have a favorable cancer outcome than those with a sustained course (100% of 8 vs. 67% of 15), but this did not reach statistical significance. Of 7 patients who were treated with DMARD or biologic, cancer outcomes included 6 who had no evidence of disease or have stable disease. Of the 8 patients who received combination ICI, 5 had a sustained course. Five patients did not have enough follow up data to define their arthritis course.
Conclusion: Our case series shows that patients can develop IRAE arthritis beyond six months of ICI therapy and that later onset IRAE arthritis trends towards a sustained arthritis course. Patients with a resolved IRAE arthritis course were more likely to have a favorable cancer outcome. IRAE interrupting cancer treatment may contribute to our observations and warrants further study into identifying predictors of IRAE development. Additionally, a concern about treating IRAE with immunosuppression is that treatment could diminish the effects of cancer therapy. However, many patients treated for IRAE arthritis in this series had favorable cancer outcomes.
References:
1. Cappelli LC et al. Arthritis Care Res. 2017;69(11):1751–1763.
2. Jamal S et al. J Rheumatol. 2020;47(2):166–175.
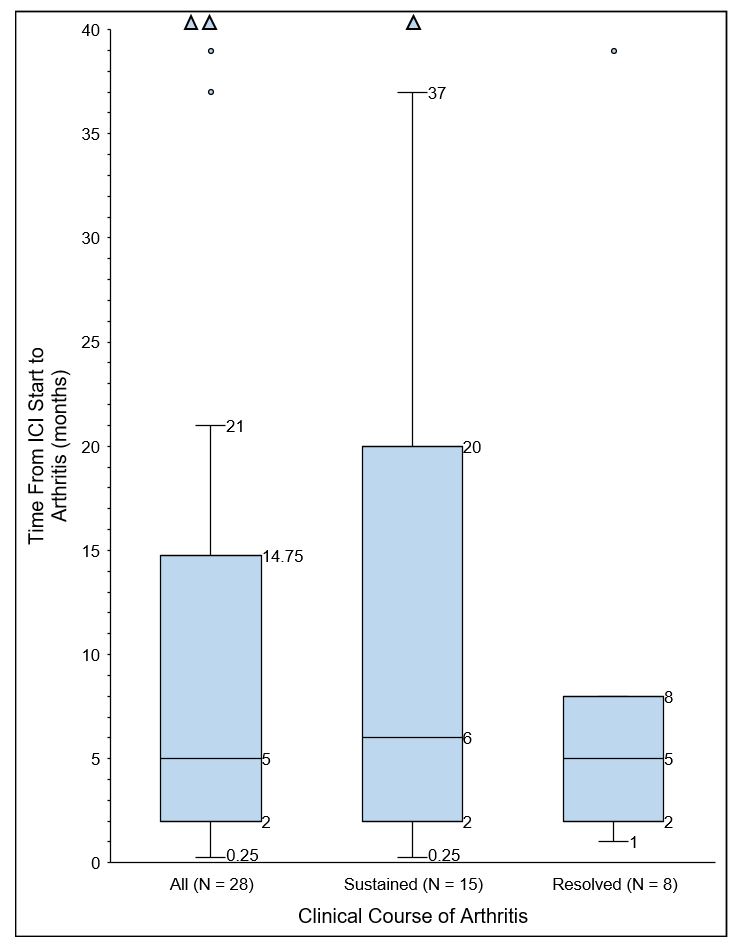 Figure 1. Delay from Immune Checkpoint Inhibitor Start to Arthritis
Figure 1. Delay from Immune Checkpoint Inhibitor Start to Arthritis
 Figure 2. Frequency of Anatomic Sites of IRAE Arthritis Symptoms
Figure 2. Frequency of Anatomic Sites of IRAE Arthritis Symptoms
To cite this abstract in AMA style:
Boutsicaris C, Ciliberti A, Lockerman E, Siddique F, Ostrowski R. Onset and Disease Course of Inflammatory Arthritis in Patients Receiving Immune Checkpoint Inhibitor Therapy at a Single Institution [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/onset-and-disease-course-of-inflammatory-arthritis-in-patients-receiving-immune-checkpoint-inhibitor-therapy-at-a-single-institution/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/onset-and-disease-course-of-inflammatory-arthritis-in-patients-receiving-immune-checkpoint-inhibitor-therapy-at-a-single-institution/