Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Combination therapy with a biologic and methotrexate (MTX) usually yields better outcomes than biologic monotherapy in rheumatoid arthritis (RA).1,2,3 However, patients may be intolerant to MTX, or prefer fewer medications if doing well. As well, some data suggest monotherapy with etanercept (ETN) may be sufficient.4 The objective of this open label, RA trial was to determine if withdrawing MTX after 6 months of combination ETN+MTX, in MTX inadequate responders, is as effective as continuing ETN+MTX.
Methods:
TNF inhibitor naïve, RA patients with active disease (≥3 swollen joints, DAS28≥3.2) despite stable MTX therapy (≥15 mg/wk, or 10 mg/wk if intolerant) for >12 weeks were enrolled. Combination therapy with ETN (50 mg/wk sc)+MTX was initiated for 6 months, followed by randomization of the patients to either continue with ETN+MTX or switch to ETN monotherapy for an additional 18 months. The primary end point was to show non-inferiority of ETN vs ETN+MTX, based on the change in DAS28-ESR, 6 months after randomization (non-inferiority margin in DAS28-ESR (-0.6)), with pre-specified analyses of subsets by disease activity (DAS28<3.2 vs DAS28≥3.2).
Results:
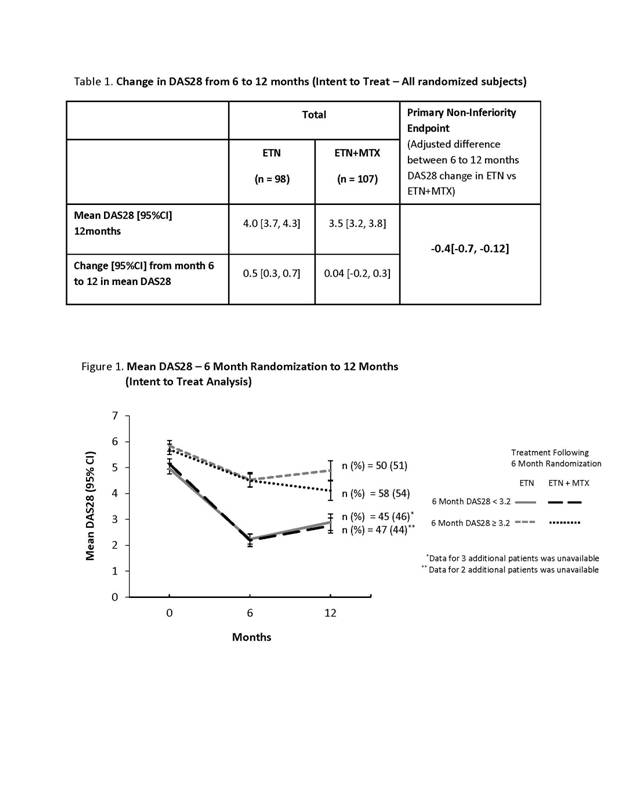
258 patients with active RA were enrolled (76% female; mean age 54.7±12.5 yrs; DAS28 5.4±1.1; duration of RA 8.9±8.4 yrs) . Mean duration of prior MTX treatment was 4.9±4.7 yrs; 48.4% of patients used ≥2 prior DMARDs and 43.8% used sc MTX. From baseline to 6 months, disease activity improved, with a change in mean DAS28 [95% CI] of -1.9 [-2.1, -1.7]. At 6 months, 205 patients were randomized with similar baseline characterisitics. Of 53 non-randomized patients, 40% discontinued treatment due to disease progression, 26% due to adverse events and 34% for other reasons. From 6 to 12 months, DAS28 was maintained in patients on ETN+MTX and increased slightly in patients on ETN monotherapy, (Table1). The primary endpoint of non-inferiority was not achieved. However, if a low disease activity (LDA) was achieved (DAS28<3.2) at 6 months, the change in DAS28 from 6 to 12 months was similar for ETN+MTX and ETN (Figure 1). Conversely, for patients on ETN+MTX with DAS28≥3.2 at 6 months disease activity continued to improve from 6 to 12 months, while patients on ETN monotherapy had slightly iincreased disease activity at 12 months (Figure 1).
Conclusion:
Patients who achieve DAS28<3.2 by 6 months on ETN+MTX have similar disease activity at 12 months, whether they continue or stop MTX. It is possible to discontinue MTX in the subset of patients who reach LDA, while it is preferable to continue MTX in those who do not achieve LDA.
References:
1. Breedveld FC, et al. Arthritis Rheum. 2006;54(1):26-37.
2. Klareskog L, et al. Lancet. 2004;363(9410):675-81.
3. van Vollenhoven RF, et al. Arthritis Res Ther. 2003;5(6):R347-51. Epub 2003 Oct 1.
4. van Riel PLCM, et al. Ann Rheum Dis. 2006;65(11):1478-83. Epub 2006 Feb 7.
Disclosure:
J. E. Pope,
Amgen,
2,
Amgen and Pfizer,
5;
B. Haraoui,
Abbott, Amgen, Bristol-Myers Squibb, Merck, Pfizer, Roche, UCB,
2,
Abbott, Amgen, Bristol-Myers Squibb, Merck, Pfizer, Roche, UCB,
9,
Abbott, Amgen, Bristol-Myers Squibb, Merck, Pfizer, Roche, UCB,
8;
J. C. Thorne,
Amgen, Pfizer, Abbott, Bristol-Myers Squibb, Roche, UCB, Merck,
2,
Amgen, Pfizer, Abbott, Bristol-Myers Squibb, Roche, UCB, Merck,
5,
Centocor, Inc.,
9;
M. Poulin-Costello,
Amgen,
3;
A. Vieira,
Amgen,
3;
E. Keystone,
Abbott Laboratories, Amgen Inc; AstraZeneca Pharmaceuticals LP, Bristol-Meyers Squibb, Centocor Inc, F. Hoffmann-LaRoche Inc, Genzyme, Merck, Novartis Pharmaceuticals, Pfizer Pharmaceuticals, UCB,
2,
Abbott Laboratories, AstraZeneca Pharmaceuticals LP, Biotest, Bristol-Meyers Squibb, Centocor Inc, F. Hoffmann-LaRoche Inc, Genentech Inc, Merck, Nycomed, Pfizer Pharmaceuticals, UCB,
5,
Abbott Laboratories, Bristol-Meyers Squibb, F. Hoffmann-LaRoche Inc, Merck, Pfizer Pharmaceuticals, UCB,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/one-year-results-from-the-canadian-methotrexate-and-etanercept-outcome-study-a-randomized-trial-of-etanercept-and-methotrexate-versus-etanercept-alone-in-rheumatoid-arthritis/