Session Information
Session Type: Abstract Session
Session Time: 4:45PM-5:00PM
Background/Purpose: Squamous and basal cell carcinomas are the most common malignancies of the skin; they are subsumed under non-melanoma skin cancer (NMSC). The risk of NMSC is increased with sun exposure. Whether treatment with certain DMARDs influences their occurrence is not clear. A study of Swedish registry data reported an increased risk of NMSC in patients with rheumatoid arthritis (RA) treated with abatacept, calling for replication (1). Using data from the German RABBIT registry, we studied the risk of NMSC under different DMARD therapies.
Methods: Data from patients with RA who were enrolled in RABBIT with the start of a biologic (b), conventional synthetic (cs) or targeted synthetic (ts) DMARD treatment were analyzed. Patients enrolled between January 2007 and October 2020 with at least one follow-up visit were included. Crude incidence rates of NMSC under different treatments using an induction period of 3 months were determined. Adjusted hazard ratios (HRs) of NMSC were calculated using Cox regression. Further adjustment for factors influencing treatment decision (“confounding by indication“) was performed using inverse probability weighting (IPW).
Results: A total of 13,870 patients were included in the analysis. There were 17 squamous cell carcinomas (0.12%) and 120 basal cell carcinomas (0.85% of patients) reported. Clear differences in patient characteristics at the start of each treatment (table 1) were seen between the classic first-line bDMARDs (tumour necrosis factor (TNF) inhibitors) and the second-line bDMARDs, with longer disease duration, significantly more severe functional limitations, higher number of prior treatment failures and more comorbidities. A comparison of crude incidence rates of NMSC under different DMARD treatments showed that the occurrence of NMSC was significantly more frequent under abatacept than under TNF inhibitors (figure).
In the adjusted Cox regression, a significantly increased risk of NMSC was observed with abatacept treatment (HR: 2.00 [95% confidence interval, CI: 1.28-3.11]) compared to csDMARD treatment. Furthermore, increasing age and number of comorbidities were significantly associated with a higher risk of NMSC occurrence (table 2). In the analysis performed with IPW, the effect for abatacept was amplified (HR: 2.18 [95% CI: 1.30-3.65]), with age and number of comorbidities also remaining significantly associated with a higher risk of NMSC (table 2).
Conclusion: This analysis of a prospective cohort study investigated the incidence rates and risk of NMSC in RA patients treated with different modes of action. Basal cell and squamous cell carcinomas were most commonly reported with abatacept treatment. Even after adjusting for differences in patient characteristics, there was a significantly increased risk of NMSC in patients treated with abatacept. It should be noted that patients initiated abatacept therapy had higher burden of comorbidity and considerable differences compared to all other treatment options. The higher risk of NMSC may be attributed to residual and unmeasured confounding due to higher burden of comorbidity or cumulative effect of therapies when used as a second or third line bDMARD.
 Figure 1 Crude incidence rates of non-melanoma skin cancer under different DMARD treatments
Figure 1 Crude incidence rates of non-melanoma skin cancer under different DMARD treatments
 Table 1 Characteristics of patients (Nf13,870) at the beginning of a therapy episode
Table 1 Characteristics of patients (Nf13,870) at the beginning of a therapy episode
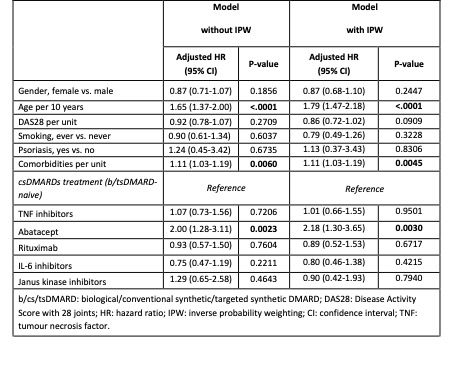 Table 2 Risk of non-melanoma skin cancer: results of multivariable Cox regression analysis with and without inverse probability weighting (IPW). Therapies were analysed with an ever-exposed approach using an induction period of 3 months.
Table 2 Risk of non-melanoma skin cancer: results of multivariable Cox regression analysis with and without inverse probability weighting (IPW). Therapies were analysed with an ever-exposed approach using an induction period of 3 months.
To cite this abstract in AMA style:
Redeker I, Herzer P, Kühne C, Schwarze I, Schaefer M, Strangfeld A. Occurrence of Basal Cell and Squamous Cell Carcinomas of the Skin Under Different DMARD Therapies [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/occurrence-of-basal-cell-and-squamous-cell-carcinomas-of-the-skin-under-different-dmard-therapies/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/occurrence-of-basal-cell-and-squamous-cell-carcinomas-of-the-skin-under-different-dmard-therapies/
