Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Axial spondyloarthritis (axSpA) is characterized by inflammation in the spine and sacroiliac joints, but can also manifest as inflammation at extra-spinal sites, most commonly inflammation of the uvea (uveitis).1 Here we aim to estimate the incidence of uveitis flares in patients (pts) with axSpA following certolizumab pegol (CZP) treatment in the RAPID-axSpA trial.
Methods: RAPID-axSpA (NCT01087762)2 was double-blind and placebo (PBO)-controlled to Week (Wk) 24, dose-blind to Wk48 and open-label to Wk204. Pts fulfilled ASAS criteria and had active axSpA, including ankylosing spondylitis (AS) and non-radiographic (nr-)axSpA. Pts were randomized 1:1:1 to either CZP 200mg Q2W, 400mg Q4W (following 400mg loading dose [LD] at Wks 0, 2, 4) or PBO. PBO pts entering dose-blind phase were re-randomized to CZP LD followed by CZP 200mg Q2W or 400mg Q4W after Wk24 or, for non-responders, Wk16. Uveitis events were recorded on extra-articular manifestation forms or adverse event forms (preferred term “Uveitis”). Events were analyzed in pts with or without a history of uveitis (defined using standard medical history, ASAS classification criteria screening assessment and baseline extra-articular assessment). At Wk24, combined CZP dosing regimens were compared with PBO; for Wk96 analyses all pts exposed to CZP were considered. Incidence rates (IR) are reported per 100 pt-yrs (PY) with censoring at time of event. No analyses of statistical significance were carried out on these data.
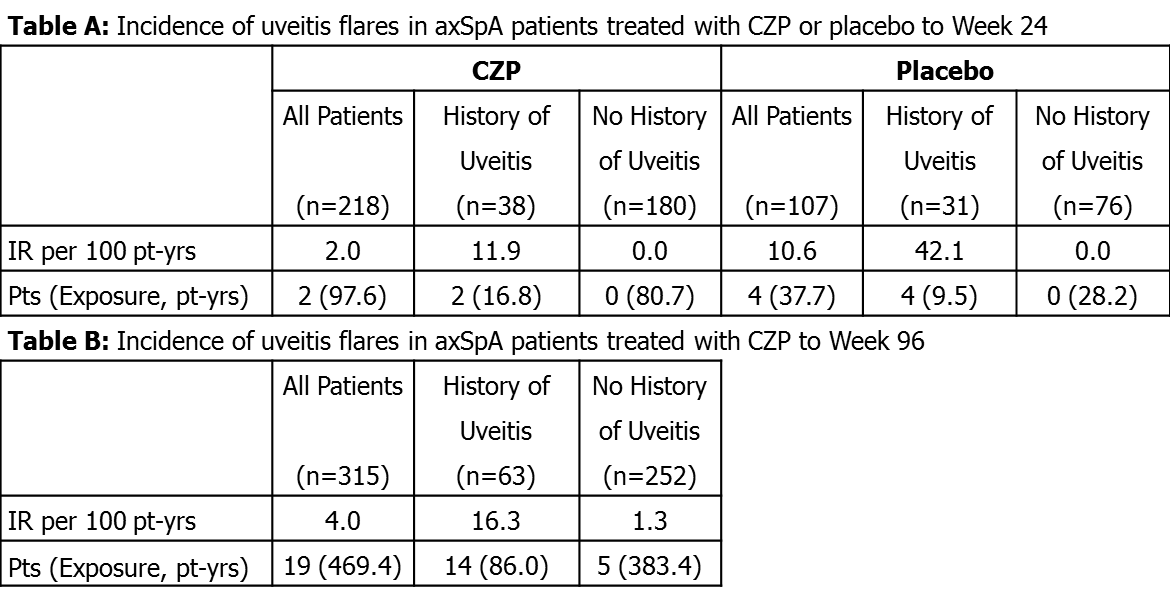
Results: At baseline, 38/218 (17.4%) CZP-randomized pts had a history of uveitis, as did 31/107 (29.0%) PBO-randomized pts. The proportion of pts with history of uveitis was similar in AS (20.8%) and nr-axSpA (21.1%) subpopulations. During the 24-wk double-blind phase, overall IR of uveitis flares (regardless of previous history) was lower in CZP pts (IR=2.0/100 PY) than PBO pts (IR=10.6/100 PY). There were no de novo cases of uveitis flares observed to Wk24 – ie. all cases were observed in pts with history of uveitis; in these pts, IR was 11.9/100 PY for CZP and 42.1/100 PY for PBO (Table A). To Wk96, 22 uveitis flares occurred in 19 pts, 11 in AS pts and 11 in nr-axSpA pts. The IR of uveitis flares (regardless of prior history of uveitis) remained low to Wk96 in CZP-treated pts (IR=4.0/100 PY; Table B). The incidence of uveitis flares in CZP-treated pts (IR=4.0/100 PY) was comparable to rates observed for other anti-TNFs in AS pts including adalimumab (IR=6.9/100 PY)3 and etanercept (IR=6.7/100 PY).4
Conclusion: The IR of uveitis flares was lower for axSpA pts treated with CZP than with PBO during the randomized controlled phase, and was comparable to the rate reported for AS pts receiving anti-TNF therapy.
References:
1. Braun J. Arthritis Rheum 2005;52(8):2447-2451
2. Landewé R. Ann Rheum Dis 2014;73:39-47
3. Rudwaleit M. Ann Rheum Dis 2009;68:696-701
4. Sieper J. Ann Rheum Dis 2010;69:226-229
Disclosure:
J. T. Rosenbaum,
Allergan, Genentech, Abbvie, UptoDate, Xoma, Santen, Sanofi, Teva, Novartis,
5;
M. Rudwaleit,
Abbott, BMS, MSD, Pfizer, Roche, UCB Pharma. ,
5;
R. B. M. Landewé,
Abbott, Ablynx, Amgen, Astra-Zeneca, Bristol Myers Squibb, Centocor, Glaxo-Smith-Kline, Novartis, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth,
5,
Abbott, Amgen, Centocor, Novartis, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth,
2,
Abbott, Amgen, Bristol Myers Squibb, Centocor, Merck, Pfizer, Roche, Schering-Plough, UCB Pharma, Wyeth,
8;
H. Marzo-Ortega,
UCB Pharma,
5;
J. Sieper,
Abbott, Merck, Pfizer, UCB Pharma, Novartis, Lilly, Janssen,
5,
Abbott, Merck, Pfizer, UCB Pharma, Novartis, Lilly, Janssen,
8;
D. M. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Vertex,
5,
Imaging Rheumatology bv,
9;
O. Davies,
UCB Pharma,
3,
UCB Pharma,
1;
C. Stach,
UCB Pharma,
3,
UCB Pharma,
1;
T. Nurminen,
UCB Pharma,
3;
A. A. Deodhar,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
2,
Abbott, Amgen, Janssen, Novartis, Pfizer and UCB Pharma,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/observed-incidence-rates-of-uveitis-over-96-weeks-of-certolizumab-pegol-treatment-in-patients-with-axial-spondyloarthritis/