Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: B-cell depletion with anti-CD20 therapy is the current cornerstone of management in organ- or life-threatening ANCA-associated vasculitis (AAV). Guidelines recommend the use of rituximab or cyclophosphamide for remission induction and rituximab for remission maintenance. Compared with rituximab, obinutuzumab is a newer humanized type 2 anti-CD20 monoclonal antibody and is postulated to result in deeper tissue B cell depletion. It has an emerging role in rituximab-intolerant patients and in rituximab-refractory disease. To date, only nine cases in two case series have been published on the use of obinutuzumab in rituximab-intolerant patients with AAV.
Methods: This single-centre case series reports the clinical outcomes of five patients who received obinutuzumab therapy for refractory or relapsing PR3+ granulomatosis with polyangiitis (GPA). All patients satisfied the 2022 ACR/EULAR classification criteria for GPA. Review of electronic health records was undertaken for patient demographics, disease manifestations, previous treatments, indication for obinutuzumab, clinical and laboratory outcomes, and potential complications.
Results: One male and four female patients with refractory or relapsing GPA received obinutuzumab. Three had multi-system involvement while one had isolated episcleritis/scleritis and another, severe isolated ENT disease. All patients had refractory or relapsing disease despite guideline-directed induction therapy with rituximab and cyclophosphamide. For induction of disease remission, obinutuzumab was administered at one or two doses of 1000mg at week 0 and week 2. Maintenance treatment consisted of single 1000mg infusions at six- to twelve- monthly intervals. Obinutuzumab was effective in inducing and maintaining disease remission. Those patients with detectable ANCA titres at time of obinutuzumab treatment seroconverted from positive to negative. During a median follow-up of 14 months (range: 4-51 months), obinutuzumab was well tolerated with two infections noted (sinusitis and moderate SARS-CoV-2 infection requiring prolonged antiviral treatment).
Conclusion: This case series illustrating the use of obinutuzumab in five patients with GPA adds to the limited published literature. Obinutuzumab shows promise in the management of rituximab-refractory or relapsing AAV and appears to be efficacious and well tolerated. Results of use thereof in larger clinical studies are awaited.
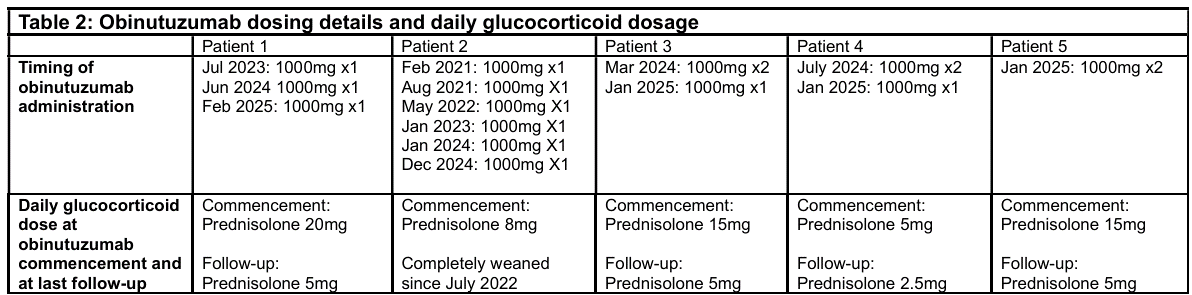 Table 1: Patient demographics, disease manifestations, indications for obinutuzumab, and treatment history
Table 1: Patient demographics, disease manifestations, indications for obinutuzumab, and treatment history
.gif) Table 2: In refractory/ relapsing AAV, obinutuzumab use facilitated glucocorticoid reduction to ≤ 5mg prednisolone daily
Table 2: In refractory/ relapsing AAV, obinutuzumab use facilitated glucocorticoid reduction to ≤ 5mg prednisolone daily
To cite this abstract in AMA style:
Au H, Löffler C, Mahrhofer H, Walz B, Hellmich B. Obinutuzumab Induces and Maintains Remission in Refractory or Relapsing ANCA-Associated Vasculitis – A Case Series [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/obinutuzumab-induces-and-maintains-remission-in-refractory-or-relapsing-anca-associated-vasculitis-a-case-series/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/obinutuzumab-induces-and-maintains-remission-in-refractory-or-relapsing-anca-associated-vasculitis-a-case-series/
