Session Information
Session Type: Late-Breaking Abstract Poster Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Fibromyalgia (FM) is a chronic, debilitating disease typified by widespread musculoskeletal pain and accompanied by fatigue, sleep disturbance, memory issues, and mood disorders. FM has been described as the prototypical centralized chronic pain syndrome. NYX-2925 is a novel, non-opioid small-molecule modulator of the N-methyl-D-aspartate receptor (NMDAR) that is in Phase 2 development for fibromyalgia and painful diabetic peripheral neuropathy.
Methods: An exploratory Phase 2, placebo-controlled neuroimaging clinical trial was conducted in 22 right-handed female subjects with FM according to ACR 2010 criteria. Following a screening period, subjects received two weeks of placebo once-daily, followed by two weeks of 20 mg NYX-2925 once-daily, followed by two weeks of 200 mg NYX-2925 once-daily. Subjects underwent resting state functional connectivity magnetic resonance imaging (rs-fcMRI) and proton magnetic resonance spectroscopy (1H-MRS) during screening and during the second week of each of the three dosing periods. Subjects and neuroimaging analysts were blinded to study drug and dose sequence.
Results: Neuroimaging showed that compared to placebo, administration of NYX-2925 resulted in statistically significant reductions of Glx/tCr (glutamate and glutamine ratio to total creatine) levels in key pain-regulating brain regions, including the dorsal anterior cingulate cortex (dACC) at rest (Placebo: 2.167+/-0.178; 20mg NYX-2925: 2.055+/-0.197; p=0.032) and the posterior insular cortex following an evoked pain stimulus (Placebo: 1.993+/-0.140; 200mg NYX-2925: 1.903+/-0.162; p=0.039). Greater concentrations of pain-evoked Glx pre-treatment in the posterior insular cortex were associated with greater reductions in patient reported sensitivity to evoked pain (increased pressure pain threshold) following 20mg NYX-2925 (r=0.58; p=0.009). In addition, 20mg NYX-2925 administration resulted in reduced connectivity between brain regions that are known to be associated with the processing of centralized chronic pain, including dACC to primary somatosensory cortex connectivity (p=0.030 Family Wise Error [FWE]).
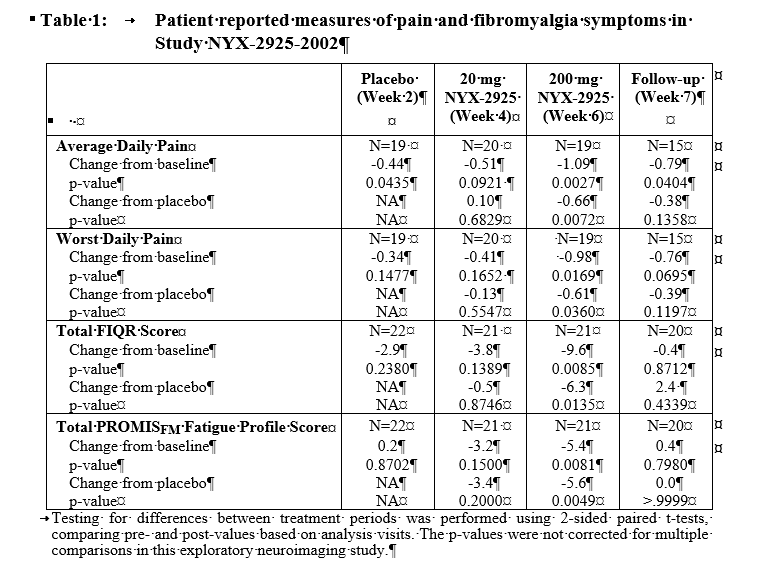
Clinically meaningful and statistically significant clinical improvements were observed following treatment with 200mg NYX-2925 compared to baseline (Week 0) and placebo (Week 2) on the average daily pain score, worst daily pain score, total Revised Fibromyalgia Impact Questionnaire (FIQR) score, and PROMISFM fatigue profile total score (Table 1). Clinical data further demonstrate that NYX-2925 is safe and well tolerated with no discontinuations due to treatment-emergent adverse events.
Conclusion: In this exploratory study, NYX-2925 demonstrated antinociceptive activity in neuroimaging evaluations. Clinically meaningful and statistically significant improvements were also observed in pain, fatigue, the overall intensity of fibromyalgia symptoms, and their impact on function. These data provide support for further evaluation of NYX-2925 for treating the hallmark symptoms of fibromyalgia.
To cite this abstract in AMA style:
Harte S, Arnold L, Ichesco E, Crumb C, Suh M, Sindelar L, Kaplan C, Larkin T, Schrepf A, Clauw D, Sainati S, Harris R. NYX-2925 Impacts Functional and Chemical Neuroimaging Biomarkers and Patient-reported Outcomes of Pain in Patients with Fibromyalgia [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/nyx-2925-impacts-functional-and-chemical-neuroimaging-biomarkers-and-patient-reported-outcomes-of-pain-in-patients-with-fibromyalgia/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nyx-2925-impacts-functional-and-chemical-neuroimaging-biomarkers-and-patient-reported-outcomes-of-pain-in-patients-with-fibromyalgia/