Session Information
Date: Tuesday, November 10, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Oxidative stress and
free radical formation play an important pathogenic role in rheumatoid
arthritis (RA) and a number of other inflammatory diseases. Oxidative stress
promotes the formation of malondialdehyde-acetaldehyde (MAA) protein adducts, leading
to robust inflammatory responses and tolerance loss. We have previously
demonstrated that MAA adducts are markedly over-expressed in inflammatory
synovitis and that anti-MAA immune responses are strongly associated with both anti-citrullinated
protein antibody (ACPA) and RA disease activity. Whether disease-modifying
therapies used to treat RA impact MAA adduct formation or exhibit free radical scavenging
properties is unknown. In this study, we sought to examine whether the
prevention of MAA adduct formation or free radical scavenging could represent
novel mechanisms of action for RA treatments, utilizing both methotrexate (MTX)
and doxycycline (DOX).
Methods: Molar equivalents of MTX or DOX were
incubated in the presence of acetaldehyde, malondialdehyde (substrates in MAA
formation) and albumin at pH 3.0, 5.0, and 7.0 for 1-3 days at 370C.
Fluorescent activity of MAA was measured at 398 nm to quantify adduct formation.
To measure free radical levels in the above reactions, electron paramagnetic
resonance (EPR) spectroscopy and the EPR spin probe 1-hydroxy-3-methoxycarbonyl-2,
2, 5, 5-tetramethylpyrrolidine (CMH) were utilized.
Results: Incubation of human albumin with MDA
and AA resulted in MAA-adduction of 8 x 104 fluorescent units (FU).
MAA adduct formation was significantly reduced in the presence of both MTX (5.6
x 104 FU; P<0.05) and DOX (1.5 x 103 FU; P<0.001). When
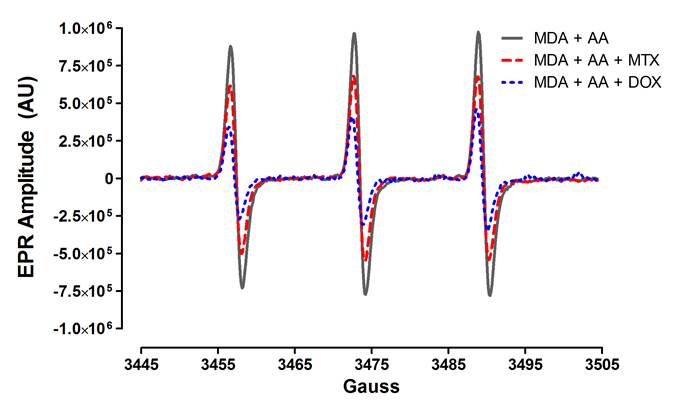
these reactions were evaluated for free radical levels by EPR, DOX and MTX
reduced the levels of free radicals generated by MDA and AA at the different pH
levels tested. For example, at pH 5 .0 MDA + AA resulted in 1.74×106
EPR arbitrary units (AU). In contrast, addition of MTX (1.23 x106
EPR AU) or DOX (7.19×105 EPR AU) to the reaction significantly
decreased the free radical levels by 30% and 41%, respectively (see figure).
Conclusion: MTX and DOX significantly decreasing MAA-adduction
of proteins (human albumin in this case) in vitro is highly important
given the known effect of MAA adduction on tolerance loss and the promotion of
both humoral and cell-mediated immunity. Moreover, our data suggest that these
agents have the capacity to directly scavenge free radicals in vitro, an
effect that has not previously been reported. For the first time, these results
lend insight into novel mechanisms of action for two agents routinely used in
the treatment of RA and other inflammatory diseases. The degree to which these
agents’ disease-modifying properties are dependent on direct free radical
scavenging requires further investigation.
To cite this abstract in AMA style:
Anderson D, Duryee MJ, Zimmerman M, Tian J, Sarmiento C, Klassen LW, O'Dell JR, Thiele GM, Mikuls TR. Novel Mechanisms of Action for Methotrexate and Doxycycline: Prevention of Protein Adduct Formation and Free Radical Scavenging [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/novel-mechanisms-of-action-for-methotrexate-and-doxycycline-prevention-of-protein-adduct-formation-and-free-radical-scavenging/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-mechanisms-of-action-for-methotrexate-and-doxycycline-prevention-of-protein-adduct-formation-and-free-radical-scavenging/