Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Response to treatment in Psoriatic Arthritis (PsA) can be captured using the OMERACT PsA Magnetic Resonance Imaging Score (PsAMRIS). While reliable and valid, PsAMRIS interpretation requires a trained reader to assess inflammatory lesions on a discrete scale ranging from 0 to 3 and gives decrease values of changes which might not capture subtle changes in inflammation in small cohorts. In this study, we propose a novel computer-assisted imaging quantitative methodology to assess early response to treatment on a continuous scale and validate it by comparing its results with PsAMRIS synovitis scores.
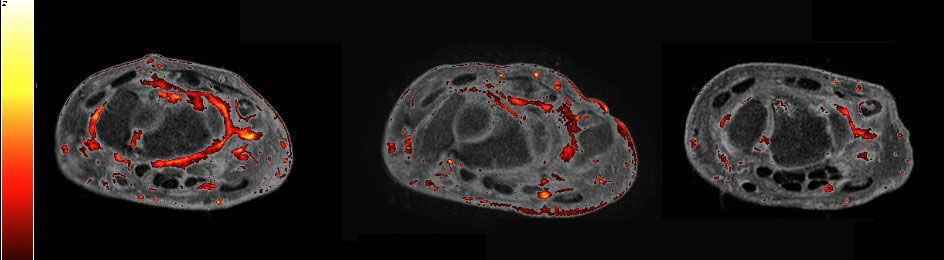
Methods: 114 patients were treated with Apremilast 30mg BID, after a 5-day titration period. Patients had MRI scans at baseline, month 3 and 6. Given the need to rapidly assess the impact of new therapies on imaging structures, PsAMRIS responses were compared to those of a new novel computer-assisted quantitative method at 3 months. Images at baseline and 3 months were scored for synovitis using the PSAMRIS interpreted by an experienced reader and were read in blinded sequences. Images at baseline, 3 and 6 months were further processed using a novel computer-assisted quantitative method. At a pixel level, each pixel’s intensity in the joint was normalized to the intensity of a reference region (muscle). A heat map of normalized intensities was produced, highlighting areas of perfusion higher than that of healthy muscle (NormI), as shown in Figure 1. An experienced reader pre-defined regions of interest (ROIs) around the wrist and the metacarpophalangeal joint (MCP) joints. From these ROIs, the volume of inflammation was calculated in each joint and tendon. This was done by counting pixels that were enhanced above the intensity level of the normal muscle. Each enhanced pixel was given a weight corresponding to the degree of enhancement, allowing to differentiate areas of residual inflammation and high perfusion. This method has been validated, tested and implemented in the CE/FDA510 cleared software package Dynamika (IAG, Image Analysis Group). Patients with non-missing data were included in the final statistical analysis.
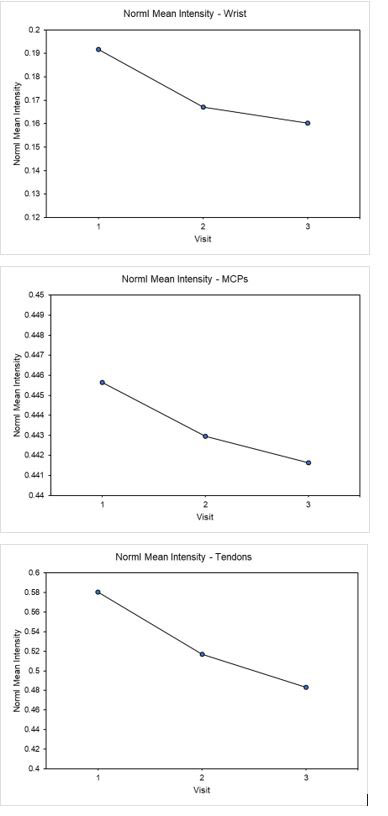
Results: IIn all cases a downward trend was observed at 3 months, indicating a reduction in inflammatory activity under treatment with Apremilast. At 3 months the NormI wrist score showed a reduction of 12% compared with the PsAMRIS synovitis score that reduced by 2%. Similar NormI results were observed for the MCPs and tendons (Figure 2). Results suggested continuous improvements over time, with reductions of 16.5% in the wrist, 0.9% in the MCPs and 16.7% in the tendons.
Conclusion: Computer assisted methodology has been developed to assess inflammation in patients with PsA and shown to be more responsive to changes in synovitis than the PsAMRIS at 3 months. Both methods demonstrated reduction of inflammation. This novel method could be used to provide early indications of treatment response. Further validation with larger datasets is planned.
To cite this abstract in AMA style:
Bird P, Boesen M, Hinton M, Sanverdi E, Hagoug R, Sabin C, Nakasato P, Guerette B, Kubassova O. Novel Computer Assisted Methodology for Quantitative Assessment of MRI Treatment Responses to Apremilast in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/novel-computer-assisted-methodology-for-quantitative-assessment-of-mri-treatment-responses-to-apremilast-in-patients-with-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-computer-assisted-methodology-for-quantitative-assessment-of-mri-treatment-responses-to-apremilast-in-patients-with-psoriatic-arthritis/