Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Sarcoidosis is a systemic inflammatory disease characterized by the presence of noncaseating granulomas. When it affects the myocardium, it can result in electrical conduction abnormalities, heart failure, and sudden death. There are no FDA-approved treatments for cardiac sarcoidosis, but the standard of care includes glucocorticoids and disease modifying anti-rheumatic drugs (DMARDs). When these therapies fail, tumor necrosis factor alpha (TNF-a) inhibitors are considered as a possible option. No formal studies have examined TNF-a inhibitors in cardiac sarcoidosis, and there is a theoretical concern that they may worsen heart failure. The aim of this retrospective review is to characterize cardiac sarcoidosis patients treated at Stanford University and to evaluate the response to TNF-a inhibitor treatment.
Methods: This is a retrospective study conducted at Stanford University. Sarcoidosis patients were identified by searching all patients seen between 2009 and 2017, ages 18 years and older, with an ICD-9/10 code for sarcoidosis, and at least one clinical note mentioning “sarcoidosis.” Each chart was manually reviewed to verify the sarcoidosis diagnosis, to determine which patients had cardiac involvement, and to extract relevant data for analysis. Continuous variables were summarized as mean and standard deviation. Categorical variables were summarized as frequencies and percentage of the total.
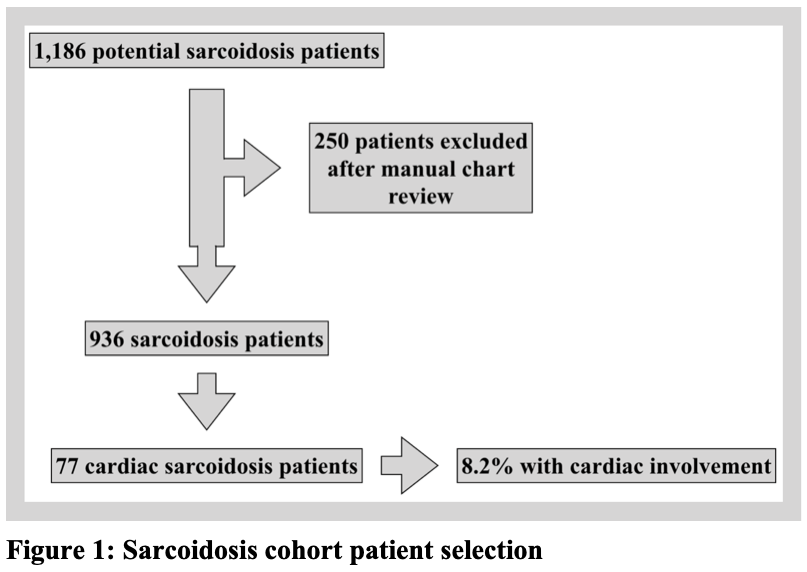
Results: A total of 1,186 potential sarcoidosis patients were identified (Figure 1), and 250 patients were excluded for not meeting diagnostic criteria. Of the 936 sarcoidosis patients remaining, 77 were identified as having cardiac sarcoidosis (8.2%). Baseline demographics (Table 1) demonstrate that the mean age at diagnosis was 55 years, 39% of patients were female, and the most common clinical presentations were heart block and tachyarrhythmia. Twenty patients (26%) received a TNF-a inhibitor (Table 2). The mean dose of prednisone before starting a TNF-a inhibitor was 23 milligrams (mg) daily, which decreased to 4 mg daily within 6 months after treatment initiation. Likewise, the mean left ventricular ejection fraction (LVEF) within one year before TNF-a inhibitor treatment was 44%, which remained stable at 47% within one year after starting treatment. This included 5 patients with an LVEF £ 30% at the time of TNF-a inhibitor initiation.
Conclusion: Twenty cardiac sarcoidosis patients in our cohort (26%) received a TNF-a inhibitor, with the overall experience being positive. Despite concerns that TNF-a inhibitors can worsen heart failure, no patients had a notable decline in LVEF within one year after treatment initiation, and most saw an improvement. This included 5 patients who had a LVEF £ 30% at the time of starting a TNF-a inhibitor. In addition, those patients who were on glucocorticoids at the time of TNF-a inhibitor initiation were all able to reduce their dose. This study supports the notion that patients with cardiac sarcoidosis who continue to have active disease despite conventional therapies can be treated with TNF-a inhibitors, and it provides a strong rationale for larger, prospective studies to be conducted in the future.
To cite this abstract in AMA style:
Baker M, Sheth K, Simard J, Shoor S, Genovese M. Novel Approach to the Treatment of Cardiac Sarcoidosis with TNF-alpha Inhibition [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/novel-approach-to-the-treatment-of-cardiac-sarcoidosis-with-tnf-alpha-inhibition/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/novel-approach-to-the-treatment-of-cardiac-sarcoidosis-with-tnf-alpha-inhibition/