Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammation is a key driver of pain in rheumatoid arthritis (RA). However, in some patients the level of pain exceeds what would be expected based on the amount of synovitis observed and acute phase reactants, which may indicate the presence of noninflammatory pain (NIP). Interleukin-6 (IL-6) has been shown in animal models to increase sensitization to pain and may play a role in NIP.
Methods: The analysis included data from three Phase 3 studies of sarilumab: MOBILITY (NCT01061736), MONARCH (NCT02332590), and TARGET (NCT01709578). Patients received double-blind placebo or sarilumab 150 mg or 200 mg subcutaneously (SC) every 2 weeks (q2w), plus weekly csDMARD (MOBILITY and TARGET), or adalimumab 40 mg or sarilumab 200 mg SC q2w as monotherapy (MONARCH). This analysis assessed the effect of sarilumab, a human IL-6 receptor inhibitor approved for the treatment of adults with moderate to severely active RA, on NIP and disease activity, stratified by baseline (BL) NIP status.
NIP was defined using an established formula: tender 28-joint count (TJC) – swollen 28-joint count (SJC) ≥7 [1,2]. Patients were assessed for NIP at study BL and for change in NIP status at Weeks 12 and 24. The proportion of patients achieving ACR20/50/70, Clinical Disease Activity Index (CDAI) ≤10, and DAS28-CRP < 3.2 at Week 24 was assessed in patients with and without BL NIP.
No inferential statistics were performed.
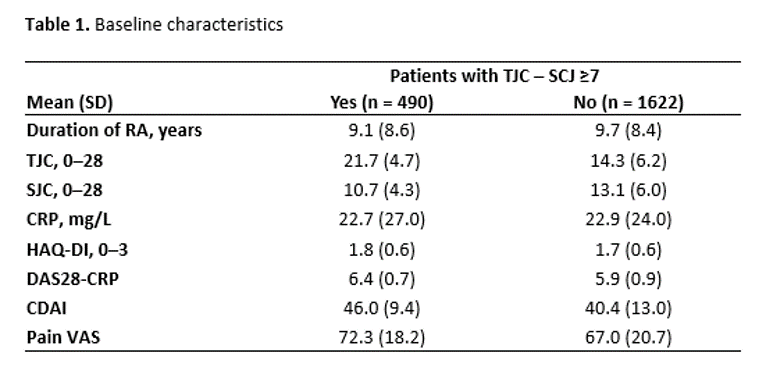
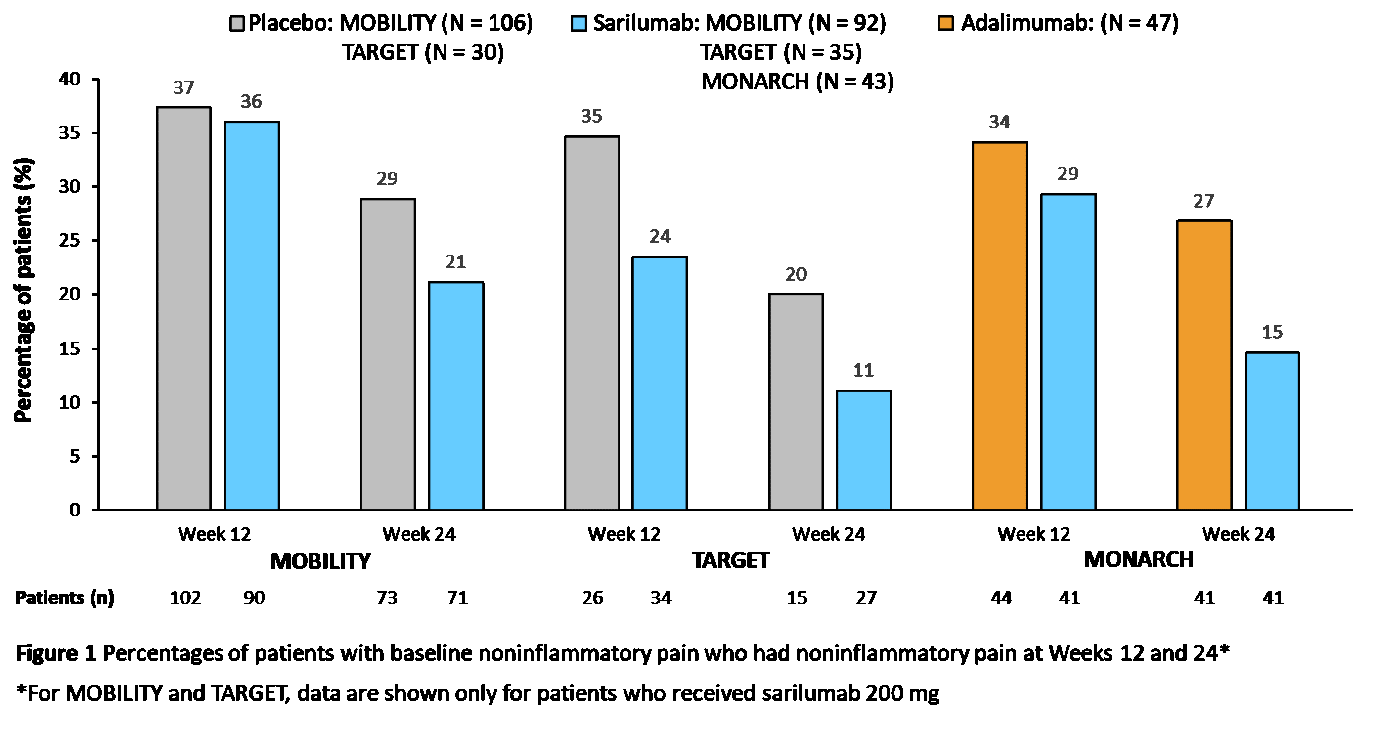
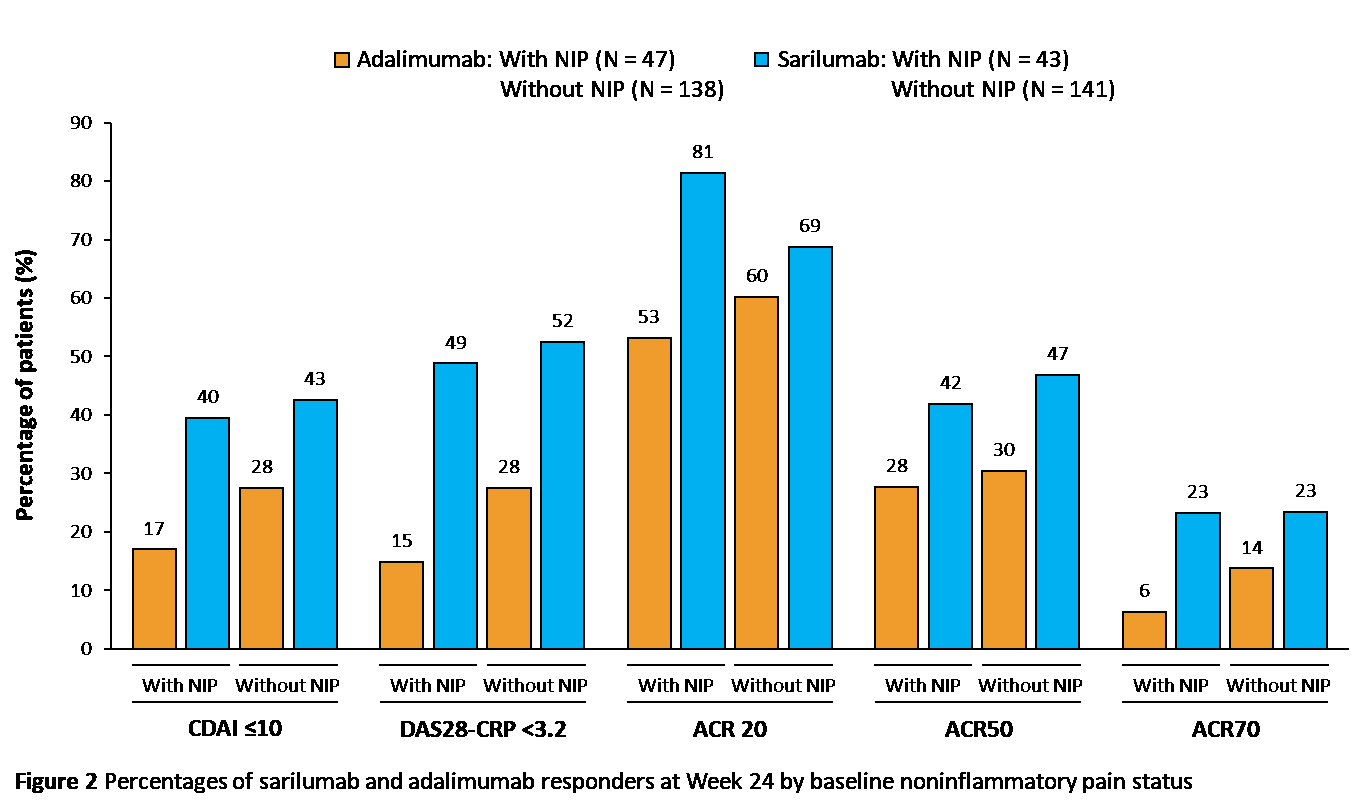
Results: Of 2112 patients in the analysis, 490 (23%) met the criteria for NIP at study BL: MOBILITY, n = 294/1197 (25%); MONARCH, n = 90/369 (24%); TARGET, n = 106/546 (19%). BL demographics were similar for patients with or without BL NIP: mean age (SD) was 52.6 (10.7) versus 51.2 (12.3) years, and 85% versus 81% were female. Patients with BL NIP had higher CDAI, DAS28-CRP, pain Visual Analog Scale (VAS), and TJC at BL versus patients without NIP (Table 1). Of patients with NIP at BL, those who received sarilumab were more likely to have no NIP at Weeks 12 and 24 versus patients who received placebo or adalimumab (Figure 1). The percentage of patients achieving improvements in disease activity at Week 24 was greater for sarilumab versus adalimumab among both patients with and without BL NIP, and these differences were larger among patients with BL NIP for all assessments except ACR50 (Figure 2).
Conclusion: NIP was prevalent at BL in the patient populations assessed. Among patients with BL NIP, a lower proportion continued to have NIP at Weeks 12 and 24 when treated with sarilumab versus placebo or adalimumab. Patients with and without BL NIP had greater improvements in pain when treated with sarilumab versus adalimumab. The difference in clinical improvement was greater among patients with BL NIP versus without BL NIP for most measures. These trends support the emerging concept that mechanisms other than direct inflammation may contribute to pain in RA, potentially mediated via IL-6 signaling.
References:
1. Durán J et al. Rheumatology. 2015;54:2166–70
2. Pollard LC et al. Rheumatology. 2010;49:924–8
To cite this abstract in AMA style:
Choy E, Bykerk V, Lee Y, St John G, van Hoogstraten H, Ford K, Praestgaard A, Sebba A. Noninflammatory Pain Is a Frequent Phenomenon in Rheumatoid Arthritis and Responds Well to Treatment with Sarilumab [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/noninflammatory-pain-is-a-frequent-phenomenon-in-rheumatoid-arthritis-and-responds-well-to-treatment-with-sarilumab/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/noninflammatory-pain-is-a-frequent-phenomenon-in-rheumatoid-arthritis-and-responds-well-to-treatment-with-sarilumab/