Session Information
Date: Monday, November 13, 2023
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: There is a paucity of data to guide biologic and JAKi DMARD selection in rheumatoid arthritis-interstitial lung disease (RA-ILD), with some reports of higher mortality with TNFi use in this population (Druce et al. RMD Open, 2017). We performed a real-world pharmacoepidemiologic study of non-TNFi/JAKi vs. TNFi in RA-ILD using the Target Trial Emulation framework (Hernan et al. JAMA, 2022).
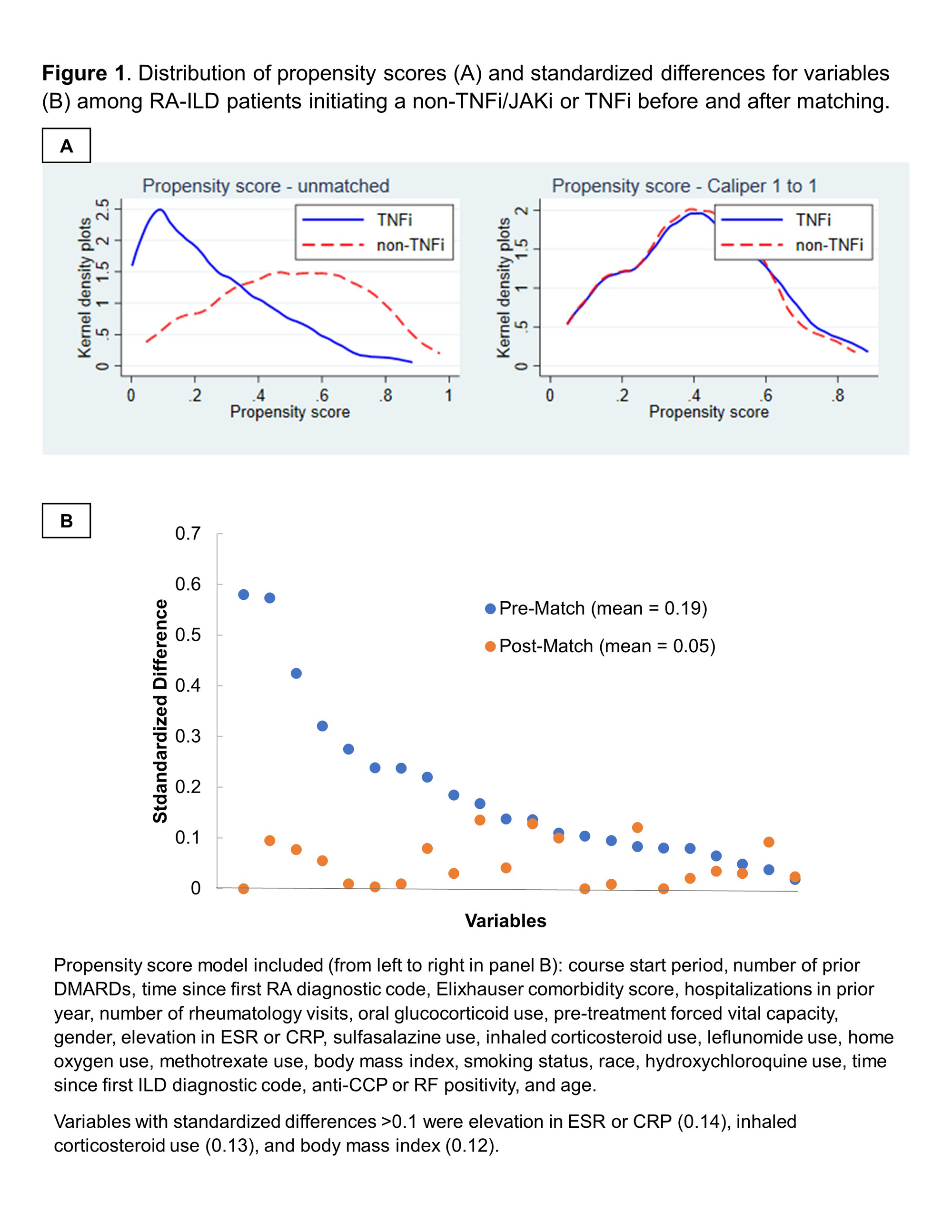
Methods: We performed an active-comparator, new-user study of patients with RA-ILD initiating TNFi or non-TNFi biologic/JAKi in the Veterans Health Administration (VA) between 2006 and 2018. Receipt of ILD-focused therapies (e.g., mycophenolate, antifibrotics) was an exclusion. RA-ILD was identified using validated administrative algorithms requiring multiple RA and ILD diagnostic codes (PPV >70%). TNFi and non-TNFi/JAKi initiators were 1:1 propensity score (PS) caliper-matched using calendar time-specific PS models (2006-2010, 2011-2015, 2016-2018) that included demographics, healthcare utilization, comorbidities, and several RA- and ILD-related factors (including pre-treatment forced vital capacity [FVC]) obtained from EHR and administrative data (Figure 1). The primary outcome was a composite of time to respiratory-related hospitalization (by VA and Medicare data linkage) or death (by National Death Index linkage) using Cox regression models following an intention-to-treat analysis approach. Secondary outcomes were respiratory hospitalization and death (all-cause, respiratory). Outcomes were assessed over 3-year (primary) and 1-year (secondary) follow-up periods. Sensitivity analyses were performed among modified cohorts requiring: 1) prior biologic/JAKi treatment, 2) non-missing pre-treatment FVC values, or 3) additional ILD classification criteria.
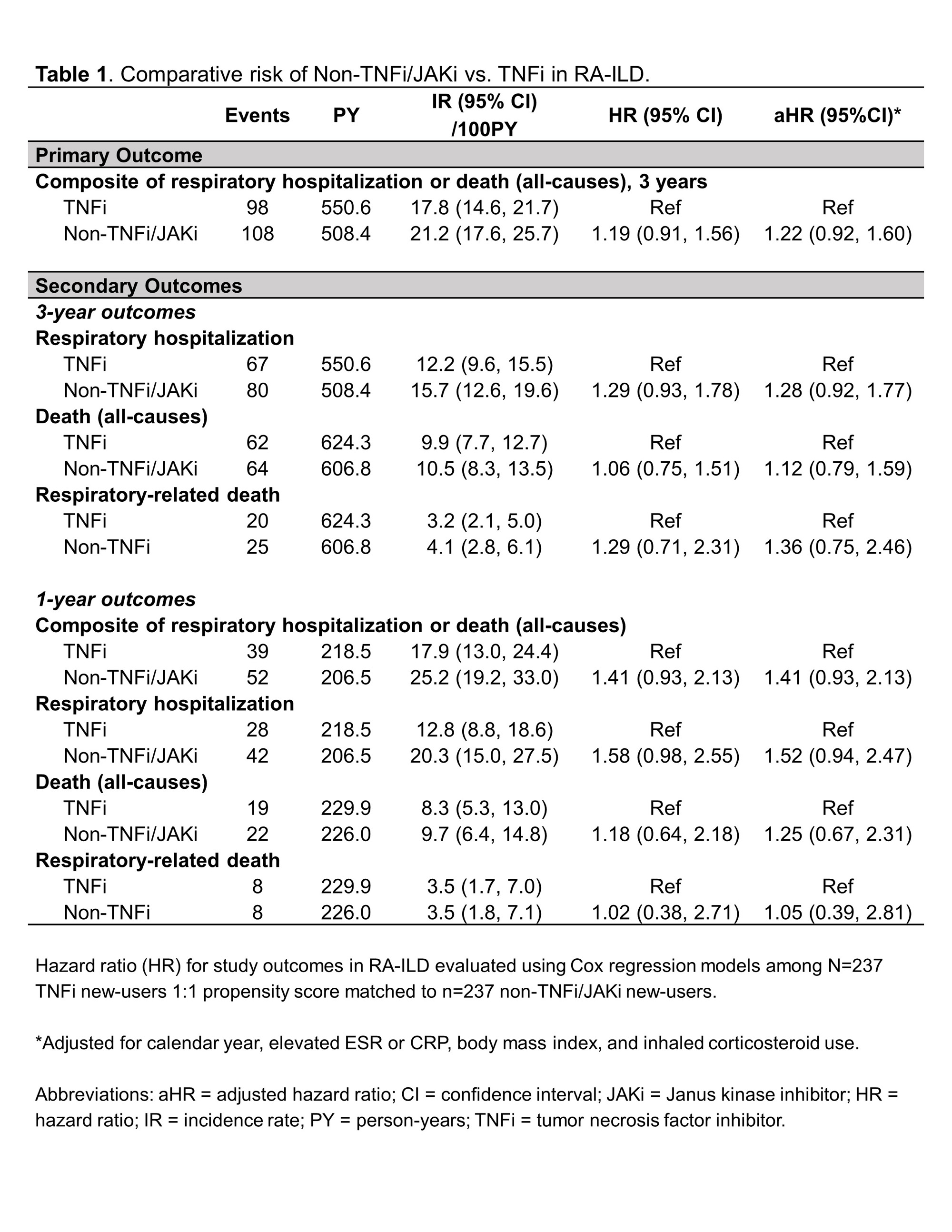
Results: Among 1,046 RA-ILD patients fulfilling eligibility criteria (n=704 TNFi), 237 TNFi initiators were matched to 237 non-TNFi/JAKi initiators in the primary analyses (mean age 68 years, 92% male). Mean standardized differences of variables in the PS model improved from 0.19 to 0.05 after matching, though few variables remained modestly imbalanced (Figure 1). Adalimumab (51%) and etanercept (37%) were the most frequent TNFi while rituximab (53%) and abatacept (28%) were the most frequent non-TNFi/JAKi. There was no significant difference in the primary outcome between non-TNFi/JAKi vs. TNFi (HR 1.19 [0.91, 1.56]; aHR 1.22 [0.92, 1.60]; Table 1). There were also no significant differences in respiratory hospitalization, all-cause mortality, or respiratory-related death (Table 1). In sensitivity analyses with modified cohort eligibility requirements, no significant differences in outcomes were observed between non-TNFi/JAKi and TNFi (Figure 2).
Conclusion: In this national VA, PS-matched study using the Target Trial Emulation framework, we did not observe significant differences in the risk of respiratory hospitalization or death between RA-ILD patients initiating non-TNFi/JAKi vs. TNFi. Our findings do not support systematic avoidance of TNFi in RA-ILD. Comparative data from clinical trials are needed to further guide these routine clinical decisions.
To cite this abstract in AMA style:
England B, Baker J, George M, Johnson T, Yang Y, Roul P, Sayles H, Yu F, Rojas Jr J, Sauer B, Cannon G, Curtis J, Mikuls T. Non-TNFi b/tsDMARDs vs. TNFi in Rheumatoid Arthritis-Interstitial Lung Disease: An Active-Comparator, New-User, Propensity Score Matched Study Using National Veterans Affairs Data [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/non-tnfi-b-tsdmards-vs-tnfi-in-rheumatoid-arthritis-interstitial-lung-disease-an-active-comparator-new-user-propensity-score-matched-study-using-national-veterans-affairs-data/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/non-tnfi-b-tsdmards-vs-tnfi-in-rheumatoid-arthritis-interstitial-lung-disease-an-active-comparator-new-user-propensity-score-matched-study-using-national-veterans-affairs-data/