Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy II: Safety and Cost Effectiveness
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: According to national guidelines issued in 2015, a non-medical switch from originator infliximab (IFX) (Remicade) to biosimilar Remsima was conducted in all Danish patients with inflammatory rheumatic diseases treated in routine care. We aimed to investigate 1) effects of the switch on serum (s) IFX and presence of anti-drug antibodies (ADA) in patients with rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axial SpA), 2) association between sIFX and ADA at the time of switch and adherence to Remsima treatment.
Methods: We included Remicade-treated patients, who switched to and were treated with Remsima. Blood samples were drawn at baseline (immediately before switching) and during follow-up (after ~3, 6, 12 months, immediately before IFX infusion to ensure trough levels). sIFX and ADA were analyzed using automated in-house assays at OUS-Radiumhospitalet. Trough sIFX<3mg/l was considered low and ≤1mg/l very low. If sIFX<5mg/l, ADA was measured. ADA≤30AU/l was considered low and ADA>30AU/l median-high. Clinical characteristics and outcomes were registered in the DANBIO registry. Remission was defined by disease activity composite scores (DAS28<2.6 (RA, PsA) and ASDAS<1.3 (axial SpA)). Medians (IQR) or percentages are shown. Comparisons were performed using Mann Whitney U test (unpaired) and Wilcoxon signed rank test (paired). The impact of baseline sIFX and ADA on treatment adherence was studied by Kaplan-Meier drug survival curves and Cox regression analyses.
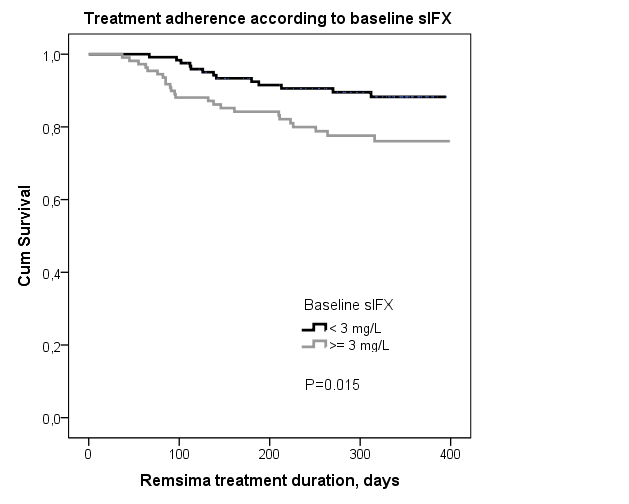
Results: 231 pts with available baseline samples from 9 departments were included (age 52 (46-65) years, 51% women). Previous IFX treatment duration was 7.1 (4.5-9.7) years. Observation time after switch was 345 (275-361) days. Trough sIFX levels increased from 2.5 mg/L at switch to 2.9 mg/L 3 months after (Table). 18 of 108 pts (17%) with low baseline sIFX had high sIFX at 3 months, and 6 of 82 pts (7%) with high baseline sIFX had low sIFX at 3 months. Presence of medium-high ADA was unchanged over time (Table) and 94% of patients had similar ADA levels at baseline and at 3 months. Concomitant methotrexate (yes/no) was not associated with baseline sIFX or ADA (both p>0.05, Mann Whitney). Patients with low sIFX (<3mg/L) received lower IFX doses than pts with sIFX≥3mg/l (i.e. 260 (200-320)mg vs. 300 (252-400)mg, p=0.005) and with longer intervals (8 (8-9)weeks vs. 7 (6-8)weeks, p<0.001). Being in remission at baseline (61%/58%/18% of RA/PsA/axial SpA patients) or at 3 months (56%/68%/19%) was not associated with sIFX (all p>0.05). A total of 37 pts (16%) stopped Remsima treatment during follow-up (lack of effect (17 pts), adverse events (11 pts), other reasons (9 pts)), median treatment duration was 126 (85-210) days. In these patients, 13/37 (35%) had low baseline s-IFX and 5 pts had ADA at baseline. ADA levels in individual patients were unchanged at the time of withdrawal of Remsima treatment. Treatment adherence was higher in patients with low baseline sIFX (<3mg/L) vs. ≥3 mg/L (Figure), also when stratified by diagnosis (RA, PsA, axial SpA. Data not shown). Presence of ADA had no impact on treatment adherence (Log rank, P=0.8).
Conclusion: In this highly selected group of patients treated with Remicade for >5 years, a non-medical switch to biosimilar Remsima had no negative impact on drug concentration or presence of ADA at 3 and 6 months following switch. At baseline, 53% of patients had low sIFX, but few patients had ADA, perhaps indicating low immunogenicity of IFX in these patients. The poorer treatment adherence among patients with high sIFX at the time of switch warrants further investigation.
| BASELINE DEMOGRAPHICS ACCORDING TO DIAGNOSIS | ||||||||
|
Rheumatoid arthritis, RA |
Psoriatic arthritis, PsA |
Axial SpA |
Other polyarthritis |
|||||
| Number of patients, n |
115 |
33 |
73 |
10 |
||||
| Remsima dose, mg/kg |
3.2 (3.0-4.6) |
4.8 (3.5-5.1) |
4.8 (3.2-5.1) |
4.2 (3.1-5.4) |
||||
| Remsima dose interval, weeks |
8 (6-8) |
7 (6-8) |
8 (6-8) |
8 (6-8) |
||||
| Concomitant methotrexate, n (%) |
89 (77%) |
14 (42%) |
13 (18%) |
3 (30%) |
||||
| S-IFX AND ADA AT BASELINE AND FOLLOW-UP. ALL DIAGNOSES | ||||||||
|
Baseline (0-45 days) |
3 months (46-135 days) |
6 months (136-286 days) |
P-values |
|||||
| Patients with available samples, n |
231 |
190 |
125* |
P1 |
P2 |
|||
| Remsima dose, mg |
300 (220-400) |
300 (220-380) |
270 (210-380) |
0.03 |
0.06 |
|||
| Trough s-Infliximab, n (%)
0-1 mg/L (very low) 1-3 mg/L (low) 3-5 mg/L (high) ≥5 mg/L (high) |
61 (26%) 61 (26%) 42 (18%) 67 (29%) |
45 (23%) 51 (27%) 28 (15%) 66 (35%) |
29 (23%) 32 (24%) 19 (15%) 45 (36%) |
0.001 |
0.5 |
|||
| Trough s-Infliximab, mg/L |
2.5 (0.9-5.9) |
2.9 (1.2-6.0) |
3.1 (1.1-6.4) |
<0.0001 |
0.3 |
|||
| ADA, n (%)
≤30 AU/L (low) >30 AU/L (median-high) Not measured (i.e. sIFX ≥ 5 mg/L) |
131 (56%) 33 (14%) 67 (29%) |
96 (51%) 28 (15%) 66 (35%) |
65 (52%) 15 (12%) 45 (36%) |
1.0 |
1.0
|
|||
| * 10 patients who stopped treatment >14 days before blood-sampling are excluded Numbers are medians (ranges) unless otherwise stated P1: Baseline vs. 3 months, P2: 3 months vs. 6 months | ||||||||
To cite this abstract in AMA style:
Glintborg B, Kringelbach TM, Høgdall E, Juul Sørensen I, Jensen DV, Loft AG, Hendricks O, Jensen Hansen IM, Linauskas A, Kristensen S, Lindegaard H, Nordin H, Bolstad N, Warren D, Gehin J, Goll GL, Lederballe Grøn K, Eng G, Enevold C, Nielsen CH, Johansen JS, Lund Hetland M. Non-Medical Switch from Originator to Biosimilar Infliximab in Patients with Inflammatory Arthritis – Impact on s-Infliximab and Antidrug-Antibodies. Results from the Danish Rheumatologic Biobank and the Danbio Registry [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/non-medical-switch-from-originator-to-biosimilar-infliximab-in-patients-with-inflammatory-arthritis-impact-on-s-infliximab-and-antidrug-antibodies-results-from-the-danish-rheumatologic-biobank-and/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/non-medical-switch-from-originator-to-biosimilar-infliximab-in-patients-with-inflammatory-arthritis-impact-on-s-infliximab-and-antidrug-antibodies-results-from-the-danish-rheumatologic-biobank-and/